Introduction
Overview
Teaching: 0 min
Exercises: 0 minQuestions
Key Points
Synapse and AD Knowledge Portal
Overview
Teaching: 45 min
Exercises: 15 minQuestions
How to work with Synapse R client?
How to work with data in AD Knowledge Portal?
Objectives
Explain how to use Synapser Package.
Demonstrate how to locate data and metadata in the Portal.
Demonstrate how to download data from the Portal programmatically.
Author: Sage Bionetworks
Setup
Install and load packages
If you haven’t already, install synapser (the Synapse R client), as well as the tidyverse family of packages.
# install synapser
install.packages("synapser", repos = c("http://ran.synapse.org", "http://cran.fhcrc.org"))
# install tidyverse if you don't already have it
install.packages("tidyverse")
We will also use the BioconductoR package manager to install biomaRt, which will help us with gene count data later.
#install.packages("XML")
if (!requireNamespace("BiocManager", quietly = TRUE))
install.packages("BiocManager")
BiocManager::install("biomaRt")
Load libraries
library(synapser)
library(tidyverse)
library(biomaRt)
Login to Synapse
Next, you will need to log in to your Synapse account.
Login option 1: Synapser takes credentials from your Synapse web session
If you are logged into the Synapse web browser, synapser will automatically use your login credentials to log you in during your R session! All you have to do is:
synLogin()
If for whatever reason that didn’t work, try one of these options:
Login option 2: Synapse username and password
In the code below, replace the <> with your Synapse username and password.
synLogin("<username>", "<password>")Login option 3: Synapse PAT
If you usually log in to Synapse with your Google account, you will need to use a Synapser Personal Access Token (PAT) to log in with the R client. Follow these instructions to generate a personal access token, then paste the PAT into the code below. Make sure you scope your access token to allow you to View, Download, and Modify.
synLogin(authToken = "<paste your personal access token here>")For more information on managing Synapse credentials with
synapser, see the documentation here.
Download data
While you can always download data from the AD Portal website via your web browser, it’s usually faster and often more convenient to download data programmatically.
Download a single file
To download a single file from the AD Knowledge Portal, you can click the linked file name to go to a page in the Synapse platform where that file is stored. Using the synID on that page, you can call the synGet() function from synapser to download the file.
Exercise 1: Use Explore Data to find processed RNAseq data from the Jax.IU.Pitt_5XFAD Study
This filters the table to a single file. In the “Id” column for this htseqcounts_5XFAD.txt file, there is a unique Synapse ID (synID).
We can then use that synID to download the file.
counts_id <- "syn22108847"
synGet(counts_id, downloadLocation = "../data/")
Bulk download files
Exercise 2: Use Explore Studies to find all metadata files from the Jax.IU.Pitt_5XFAD study
Use the facets and search bar to look for data you want to download from the AD Knowledge Portal. Once you’ve identified the files you want, click on the download arrow icon on the top right of the Explore Data table and select “Programmatic Options” from the drop-down menu.
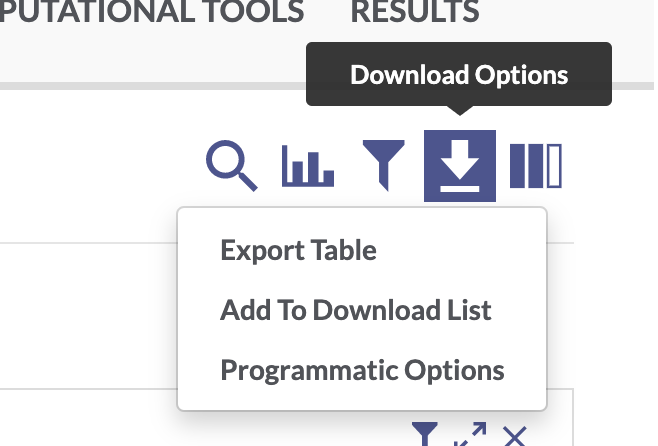
In the window that pops up, select the “R” tab from the top menu bar. This will display some R code that constructs a SQL query of the Synapse data table that drives the AD Knowledge Portal. This query will allow us to download only the files that meet our search criteria.
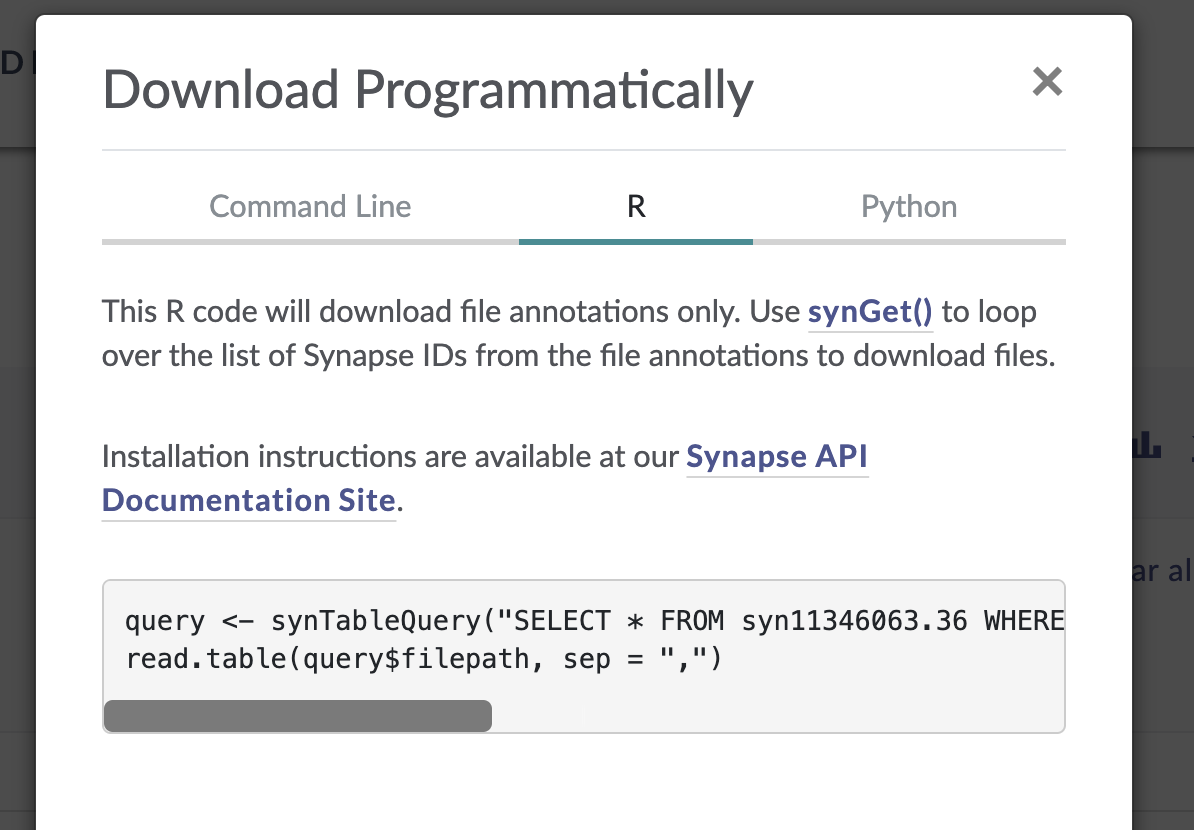
The function synTableQuery() returns a Synapse object wrapper around a CSV file that is automatically downloaded to a Synapse cache directory .synapseCache in your home directory. You can use query$filepath to see the path to the file in the Synapse cache.
# download the results of the filtered table query
query <- synTableQuery("SELECT * FROM syn11346063.37 WHERE ( ( `study` HAS ( 'Jax.IU.Pitt_5XFAD' ) ) AND ( `resourceType` = 'metadata' ) )")
# view the file path of the resulting csv
query$filepath
[1] "/Users/auyar/.synapseCache/628/125732628/SYNAPSE_TABLE_QUERY_125732628.csv"
We’ll use read.csv to read the CSV file into R (although the provided read.table or any other base R version is also fine!). We can explore the download_table object and see that it contains information on all of the AD Portal data files we want to download. Some columns like the “id” and “parentId” columns contain info about where the file is in Synapse, and some columns contain AD Portal annotations for each file, like “dataType”, “specimenID”, and “assay”. This annotation table will later allow us to link downloaded files to additional metadata variables!
# read in the table query csv file
download_table <- read_csv(query$filepath, show_col_types = FALSE)
download_table
# A tibble: 5 × 45
ROW_ID ROW_VERSION ROW_ETAG id name study dataType assay organ tissue
<dbl> <dbl> <chr> <chr> <chr> <chr> <chr> <chr> <lgl> <lgl>
1 22094731 2 84e4bc38-9… syn2… Jax.… "[\"… "[\"ima… "[\"… NA NA
2 22094732 2 266ec572-1… syn2… Jax.… "[\"… "[\"ima… "[\"… NA NA
3 22103212 3 7229ee91-4… syn2… Jax.… "[\"… <NA> <NA> NA NA
4 22103213 3 ce970c4c-d… syn2… Jax.… "[\"… <NA> <NA> NA NA
5 22110328 4 20cf3097-4… syn2… Jax.… "[\"… "[\"gen… "[\"… NA NA
# ℹ 35 more variables: species <chr>, sex <lgl>, consortium <chr>, grant <chr>,
# modelSystemName <lgl>, treatmentType <lgl>, specimenID <lgl>,
# individualID <lgl>, individualIdSource <lgl>, specimenIdSource <lgl>,
# resourceType <chr>, dataSubtype <lgl>, metadataType <chr>,
# assayTarget <lgl>, analysisType <lgl>, cellType <lgl>,
# nucleicAcidSource <lgl>, fileFormat <chr>, group <lgl>,
# isModelSystem <lgl>, isConsortiumAnalysis <lgl>, isMultiSpecimen <lgl>, …
Finally, we use a mapping function from the purrr package to loop through the “id” column and apply the synGet() function to each file’s synID. In this case, we use purrr::walk() because it lets us call synGet() for its side effect (downloading files to a location we specify), and returns nothing.
# loop through the column of synIDs and download each file
purrr::walk(download_table$id, ~synGet(.x, downloadLocation = "../data/"))
Congratulations, you have bulk downloaded files from the AD Knowledge Portal!
An important note: for situations where you are downloading many large files, the R client performs substantially slower than the command line client or the Python client. In these cases, you can use the instructions and code snippets for the command line or Python client provided in the “Programmatic Options” menu.
Working with AD Portal metadata
Metadata basics
We have now downloaded several metadata files and an RNAseq counts file from the portal. For our next exercises, we want to read those files in as R data so we can work with them.
We can see from the download_table we got during the bulk download step that we have five metadata files. Two of these should be the individual and biospecimen files, and three of them are assay metadata files.
#
download_table %>%
dplyr::select(name, metadataType, assay)
# A tibble: 5 × 3
name metadataType assay
<chr> <chr> <chr>
1 Jax.IU.Pitt_5XFAD_assay_autorad_metadata.csv assay "[\"autoradiography…
2 Jax.IU.Pitt_5XFAD_assay_PET_metadata.csv assay "[\"Positron Emissi…
3 Jax.IU.Pitt_5XFAD_individual_metadata.csv individual <NA>
4 Jax.IU.Pitt_5XFAD_biospecimen_metadata.csv biospecimen <NA>
5 Jax.IU.Pitt_5XFAD_assay_RNAseq_metadata.csv assay "[\"rnaSeq\"]"
We are only interested in RNAseq data, so we will only read in the individual, biospecimen, and RNAseq assay metadata files.
# counts matrix
counts <- read_tsv("../data/htseqcounts_5XFAD.txt", show_col_types = FALSE)
# individual metadata
ind_meta <- read_csv("../data/Jax.IU.Pitt_5XFAD_individual_metadata.csv", show_col_types = FALSE)
# biospecimen metadata
bio_meta <- read_csv("../data/Jax.IU.Pitt_5XFAD_biospecimen_metadata.csv", show_col_types = FALSE)
#assay metadata
rna_meta <- read_csv("../data/Jax.IU.Pitt_5XFAD_assay_RNAseq_metadata.csv", show_col_types = FALSE)
Let’s examine the data and metadata files a bit before we begin our analyses.
Counts data
# Calling a tibble object will print the first ten rows in a nice tidy output; doing the same for a base R dataframe will print the whole thing until it runs out of memory. If you want to inspect a large dataframe, use `head(df)`
counts
# A tibble: 55,489 × 73
gene_id `32043rh` `32044rh` `32046rh` `32047rh` `32048rh` `32049rh` `32050rh`
<chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
1 ENSG00… 22554 0 0 0 16700 0 0
2 ENSG00… 344489 4 0 1 260935 6 8
3 ENSMUS… 5061 3483 3941 3088 2756 3067 2711
4 ENSMUS… 0 0 0 0 0 0 0
5 ENSMUS… 208 162 138 127 95 154 165
6 ENSMUS… 44 17 14 28 23 24 14
7 ENSMUS… 143 88 121 117 115 109 75
8 ENSMUS… 22 6 10 11 11 19 24
9 ENSMUS… 7165 5013 5581 4011 4104 5254 4345
10 ENSMUS… 3728 2316 2238 1965 1822 1999 1809
# ℹ 55,479 more rows
# ℹ 65 more variables: `32052rh` <dbl>, `32053rh` <dbl>, `32057rh` <dbl>,
# `32059rh` <dbl>, `32061rh` <dbl>, `32062rh` <dbl>, `32065rh` <dbl>,
# `32067rh` <dbl>, `32068rh` <dbl>, `32070rh` <dbl>, `32073rh` <dbl>,
# `32074rh` <dbl>, `32075rh` <dbl>, `32078rh` <dbl>, `32081rh` <dbl>,
# `32088rh` <dbl>, `32640rh` <dbl>, `46105rh` <dbl>, `46106rh` <dbl>,
# `46107rh` <dbl>, `46108rh` <dbl>, `46109rh` <dbl>, `46110rh` <dbl>, …
The data file has a column of ENSEMBL gene ids and then a bunch of columns with count data, where the column headers correspond to the specimenIDs. These specimenIDs should all be in the RNAseq assay metadata file, so let’s check.
# what does the RNAseq assay metadata look like?
rna_meta
# A tibble: 72 × 12
specimenID platform RIN rnaBatch libraryBatch sequencingBatch libraryPrep
<chr> <chr> <lgl> <dbl> <dbl> <dbl> <chr>
1 32043rh IlluminaN… NA 1 1 1 polyAselec…
2 32044rh IlluminaN… NA 1 1 1 polyAselec…
3 32046rh IlluminaN… NA 1 1 1 polyAselec…
4 32047rh IlluminaN… NA 1 1 1 polyAselec…
5 32049rh IlluminaN… NA 1 1 1 polyAselec…
6 32057rh IlluminaN… NA 1 1 1 polyAselec…
7 32061rh IlluminaN… NA 1 1 1 polyAselec…
8 32065rh IlluminaN… NA 1 1 1 polyAselec…
9 32067rh IlluminaN… NA 1 1 1 polyAselec…
10 32070rh IlluminaN… NA 1 1 1 polyAselec…
# ℹ 62 more rows
# ℹ 5 more variables: libraryPreparationMethod <lgl>, isStranded <lgl>,
# readStrandOrigin <lgl>, runType <chr>, readLength <dbl>
# are all the column headers from the counts matrix (except the first "gene_id" column) in the assay metadata?
all(colnames(counts[-1]) %in% rna_meta$specimenID)
[1] TRUE
Assay metadata
The assay metadata contains information about how data was generated on each sample in the assay. Each specimenID represents a unique sample. We can use some tools from dplyr to explore the metadata.
# how many unique specimens were sequenced?
n_distinct(rna_meta$specimenID)
[1] 72
# were the samples all sequenced on the same platform?
distinct(rna_meta, platform)
# A tibble: 1 × 1
platform
<chr>
1 IlluminaNovaseq6000
# were there multiple sequencing batches reported?
distinct(rna_meta, sequencingBatch)
# A tibble: 1 × 1
sequencingBatch
<dbl>
1 1
Biospecimen metadata
The biospecimen metadata contains specimen-level information, including organ and tissue the specimen was taken from, how it was prepared, etc. Each specimenID is mapped to an individualID.
# all specimens from the RNAseq assay metadata file should be in the biospecimen file
all(rna_meta$specimenID %in% bio_meta$specimenID)
[1] TRUE
# but the biospecimen file also contains specimens from different assays
all(bio_meta$specimenID %in% rna_meta$specimenID)
[1] FALSE
Individual metadata
The individual metadata contains information about all the individuals in the study, represented by unique individualIDs. For humans, this includes information on age, sex, race, diagnosis, etc. For MODEL-AD mouse models, the individual metadata has information on model genotypes, stock numbers, diet, and more.
# all individualIDs in the biospecimen file should be in the individual file
all(bio_meta$individualID %in% ind_meta$individualID)
[1] FALSE
# which model genotypes are in this study?
distinct(ind_meta, genotype)
# A tibble: 3 × 1
genotype
<chr>
1 <NA>
2 5XFAD_carrier
3 5XFAD_noncarrier
Joining metadata
We use the three-file structure for our metadata because it allows us to store metadata for each study in a tidy format. Every line in the assay and biospecimen files represents a unique specimen, and every line in the individual file represents a unique individual. This means the files can be easily joined by specimenID and individualID to get all levels of metadata that apply to a particular data file. We will use the left_join() function from the dplyr package, and the %>% operator from the magrittr package. If you are unfamiliar with the pipe, think of it as a shorthand for “take this (the preceding object) and do that (the subsequent command)”. See here for more info on piping in R.
# join all the rows in the assay metadata that have a match in the biospecimen metadata
joined_meta <- rna_meta %>% #start with the rnaseq assay metadata
left_join(bio_meta, by = "specimenID") %>% #join rows from biospecimen that match specimenID
left_join(ind_meta, by = "individualID") # join rows from individual that match individualID
joined_meta
# A tibble: 144 × 50
specimenID platform RIN rnaBatch libraryBatch sequencingBatch libraryPrep
<chr> <chr> <lgl> <dbl> <dbl> <dbl> <chr>
1 32043rh IlluminaN… NA 1 1 1 polyAselec…
2 32043rh IlluminaN… NA 1 1 1 polyAselec…
3 32044rh IlluminaN… NA 1 1 1 polyAselec…
4 32044rh IlluminaN… NA 1 1 1 polyAselec…
5 32046rh IlluminaN… NA 1 1 1 polyAselec…
6 32046rh IlluminaN… NA 1 1 1 polyAselec…
7 32047rh IlluminaN… NA 1 1 1 polyAselec…
8 32047rh IlluminaN… NA 1 1 1 polyAselec…
9 32049rh IlluminaN… NA 1 1 1 polyAselec…
10 32049rh IlluminaN… NA 1 1 1 polyAselec…
# ℹ 134 more rows
# ℹ 43 more variables: libraryPreparationMethod <lgl>, isStranded <lgl>,
# readStrandOrigin <lgl>, runType <chr>, readLength <dbl>,
# individualID <dbl>, specimenIdSource <chr>, organ <chr>, tissue <chr>,
# BrodmannArea <lgl>, sampleStatus <chr>, tissueWeight <lgl>,
# tissueVolume <lgl>, nucleicAcidSource <lgl>, cellType <lgl>,
# fastingState <lgl>, isPostMortem <lgl>, samplingAge <lgl>, ...1 <dbl>, …
We now have a very wide dataframe that contains all the available metadata on each specimen in the RNAseq data from this study. This procedure can be used to join the three types of metadata files for every study in the AD Knowledge Portal, allowing you to filter individuals and specimens as needed based on your analysis criteria!
# convert columns of strings to month-date-year format
joined_meta_time <- joined_meta %>%
# convert numeric ages to timepoint categories
mutate(timepoint = case_when(ageDeath > 10 ~ "12 mo",
ageDeath < 10 & ageDeath > 5 ~ "6 mo",
ageDeath < 5 ~ "4 mo"))
covars_5XFAD <- joined_meta_time %>%
dplyr::select(individualID, specimenID, sex, genotype, timepoint) %>% distinct() %>% as.data.frame()
rownames(covars_5XFAD) <- covars_5XFAD$specimenID
We will save joined_meta for the next lesson.
saveRDS(covars_5XFAD, file = "../data/covars_5XFAD.rds")
Single-specimen files
For files that contain data from a single specimen (e.g. raw sequencing files, raw mass spectra, etc.), we can use the Synapse annotations to associate these files with the appropriate metadata.
Excercise 3: Use Explore Data to find all RNAseq files from the Jax.IU.Pitt_5XFAD study.
If we filter for data where Study = “Jax.IU.Pitt_5XFAD” and Assay = “rnaSeq” we will get a list of 148 files, including raw fastqs and processed counts data.
Synapse entity annotations
We can use the function synGetAnnotations to view the annotations associated with any file without downloading the file.
# the synID of a random fastq file from this list
random_fastq <- "syn22108503"
# extract the annotations as a nested list
fastq_annotations <- synGetAnnotations(random_fastq)
fastq_annotations
$sex
[1] "female"
$room
[1] "JAX_MGL373"
$assay
[1] "rnaSeq"
$grant
[1] "U54AG054345"
$organ
[1] "brain"
$study
[1] "Jax.IU.Pitt_5XFAD"
$tissue
[1] "right cerebral hemisphere"
$bedding
[1] "aspen"
$birthID
[1] "RMO1223"
$climbID
[1] "298456"
$species
[1] "Mouse"
$waterpH
[1] 2.85
$ageDeath
[1] 10.81967
$dataType
[1] "geneExpression"
$genotype
[1] "5XFAD_carrier"
$matingID
[1] "M-108-17"
$dateBirth
[1] "1521417600000"
$consortium
[1] "MODEL-AD"
$fileFormat
[1] "fastq"
$generation
[1] "N1F3"
$rodentDiet
[1] "0.06"
$specimenID
[1] "32043rh"
$brainWeight
[1] 0.503
$dataSubtype
[1] "raw"
$microchipID
[1] "288646853"
$stockNumber
[1] "8730"
$individualID
[1] "32043"
$officialName
[1] "B6.Cg-Tg(APPSwFlLon,PSEN1*M146L*L286V)6799Vas/Mmjax"
$resourceType
[1] "experimentalData"
$rodentWeight
[1] 28.76
$ageDeathUnits
[1] "months"
$isModelSystem
[1] FALSE
$materialOrigin
[1] "JAX"
$isMultiSpecimen
[1] FALSE
$modelSystemName
[1] "5XFAD"
$modelSystemType
[1] "animal"
$nucleicAcidSource
[1] "bulk cell"
$genotypeBackground
[1] "C57BL6J"
$individualIdSource
[1] "JAX"
$individualCommonGenotype
[1] "5XFAD"
The file annotations let us see which study the file is associated with (Jax.IU.Pitt.5XFAD), which species it’s from (Mouse), which assay generated the file (rnaSeq), and a whole bunch of other properties. Most importantly, single-specimen files are annotated with with the specimenID of the specimen in the file, and the individualID of the individual that specimen was taken from. We can use these annotations to link files to the rest of the metadata, including metadata that is not in annotations. This is especially helpful for human studies, as potentially identifying information like age, race, and diagnosis is not included in file annotations.
# find records belonging to the individual this file maps to in our joined metadata
joined_meta %>%
filter(individualID == fastq_annotations$individualID[[1]])
# A tibble: 2 × 50
specimenID platform RIN rnaBatch libraryBatch sequencingBatch libraryPrep
<chr> <chr> <lgl> <dbl> <dbl> <dbl> <chr>
1 32043rh IlluminaNo… NA 1 1 1 polyAselec…
2 32043rh IlluminaNo… NA 1 1 1 polyAselec…
# ℹ 43 more variables: libraryPreparationMethod <lgl>, isStranded <lgl>,
# readStrandOrigin <lgl>, runType <chr>, readLength <dbl>,
# individualID <dbl>, specimenIdSource <chr>, organ <chr>, tissue <chr>,
# BrodmannArea <lgl>, sampleStatus <chr>, tissueWeight <lgl>,
# tissueVolume <lgl>, nucleicAcidSource <lgl>, cellType <lgl>,
# fastingState <lgl>, isPostMortem <lgl>, samplingAge <lgl>, ...1 <dbl>,
# climbID <dbl>, microchipID <dbl>, birthID <chr>, matingID <chr>, …
Annotations during bulk download
When bulk downloading many files, the best practice is to preserve the download manifest that is generated which lists all the files, their synIDs, and all their annotations. If using the Synapse R client, follow the instructions in the Bulk download files section above.
If we use the “Programmatic Options” tab in the AD Portal download menu to download all 148 rnaSeq files from the 5XFAD study, we would get a table query that looks like this:
query <- synTableQuery("SELECT * FROM syn11346063.37 WHERE ( ( \"study\" HAS ( 'Jax.IU.Pitt_5XFAD' ) ) AND ( \"assay\" HAS ( 'rnaSeq' ) ) )")
As we saw previously, this downloads a csv file with the results of our AD Portal query. Opening that file lets us see which specimens are associated with which files:
#
annotations_table <- read_csv(query$filepath, show_col_types = FALSE)
annotations_table
# A tibble: 148 × 45
ROW_ID ROW_VERSION ROW_ETAG id name study dataType assay organ tissue
<dbl> <dbl> <chr> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
1 22108503 1 458ff182-… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
2 22108508 1 163d5067-… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
3 22108512 1 d02e16c5-… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
4 22108519 1 59eba082-… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
5 22108525 1 9fed0677-… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
6 22108530 1 47cff701-… syn2… 3205… "[\"… "[\"gen… "[\"… brain "[\"r…
7 22108534 1 03af7c0a-… syn2… 3206… "[\"… "[\"gen… "[\"… brain "[\"r…
8 22108539 1 502d1468-… syn2… 3206… "[\"… "[\"gen… "[\"… brain "[\"r…
9 22108543 1 47b8ffe9-… syn2… 3206… "[\"… "[\"gen… "[\"… brain "[\"r…
10 22108550 1 471ec9a1-… syn2… 3207… "[\"… "[\"gen… "[\"… brain "[\"r…
# ℹ 138 more rows
# ℹ 35 more variables: species <chr>, sex <chr>, consortium <chr>, grant <chr>,
# modelSystemName <chr>, treatmentType <lgl>, specimenID <chr>,
# individualID <dbl>, individualIdSource <chr>, specimenIdSource <lgl>,
# resourceType <chr>, dataSubtype <chr>, metadataType <chr>,
# assayTarget <lgl>, analysisType <lgl>, cellType <lgl>,
# nucleicAcidSource <chr>, fileFormat <chr>, group <lgl>, …
You could then use purrr::walk(download_table$id, ~synGet(.x, downloadLocation = <your-download-directory>)) to walk through the column of synIDs and download all 148 files. However, because these are large files, it might be preferable to use the Python client or command line client for increased speed.
Once you’ve downloaded all the files in the id column, you can link those files to their annotations by the name column.
# We'll use the "random fastq" that we got annotations for earlier
# To avoid downloading the whole 3GB file, we'll use synGet with "downloadFile = FALSE" to get only the Synapse entity information, rather than the file.
# If we downloaded the actual file, we could find it in the directory and search using the filename. Since we're just downloading the Synapse entity wrapper object, we'll use the file name listed in the object properties.
fastq <- synGet(random_fastq, downloadFile = FALSE)
# filter the annotations table to rows that match the fastq filename
annotations_table %>%
filter(name == fastq$properties$name)
# A tibble: 1 × 45
ROW_ID ROW_VERSION ROW_ETAG id name study dataType assay organ tissue
<dbl> <dbl> <chr> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
1 22108503 1 458ff182-d… syn2… 3204… "[\"… "[\"gen… "[\"… brain "[\"r…
# ℹ 35 more variables: species <chr>, sex <chr>, consortium <chr>, grant <chr>,
# modelSystemName <chr>, treatmentType <lgl>, specimenID <chr>,
# individualID <dbl>, individualIdSource <chr>, specimenIdSource <lgl>,
# resourceType <chr>, dataSubtype <chr>, metadataType <chr>,
# assayTarget <lgl>, analysisType <lgl>, cellType <lgl>,
# nucleicAcidSource <chr>, fileFormat <chr>, group <lgl>,
# isModelSystem <lgl>, isConsortiumAnalysis <lgl>, isMultiSpecimen <lgl>, …
Multispecimen files
Multispecimen files in the AD Knowledge Portal are files that contain data or information from more than one specimen. They are not annotated with individualIDs or specimenIDs, since these files may contain numbers of specimens that exceed the annotation limits. These files are usually processed or summary data (gene counts, peptide quantifications, etc), and are always annotated with isMultiSpecimen = TRUE.
If we look at the processed data files in the table of 5XFAD RNAseq file annotations we just downloaded, we will see that it isMultiSpecimen = TRUE, but individualID and specimenID are blank:
#
annotations_table %>%
filter(fileFormat == "txt") %>%
dplyr::select(name, individualID, specimenID, isMultiSpecimen)
# A tibble: 3 × 4
name individualID specimenID isMultiSpecimen
<chr> <dbl> <chr> <lgl>
1 htseqcounts_5XFAD.txt NA <NA> TRUE
2 tpm_gene_5XFAD.txt NA <NA> TRUE
3 tpm_isoform_5XFAD.txt NA <NA> TRUE
The multispecimen file should contain a row or column of specimenIDs that correspond to the specimenIDs used in a study’s metadata, as we have seen with the 5XFAD counts file.
# In this example, we take a slice of the counts data to reduce computation, transpose it so that each row represents a single specimen, and then join it to the joined metadata by the specimenID
counts %>%
slice_head(n = 5) %>%
t() %>%
as_tibble(rownames = "specimenID") %>%
left_join(joined_meta, by = "specimenID")
Warning: The `x` argument of `as_tibble.matrix()` must have unique column names if `.name_repair` is
omitted as of tibble 2.0.0.
ℹ Using compatibility `.name_repair`.
This warning is displayed once every 8 hours.
Call `lifecycle::last_lifecycle_warnings()` to see where this warning was generated.
# A tibble: 145 × 55
specimenID V1 V2 V3 V4 V5 platform RIN rnaBatch libraryBatch
<chr> <chr> <chr> <chr> <chr> <chr> <chr> <lgl> <dbl> <dbl>
1 gene_id "ENS… "ENS… "ENS… "ENS… "ENS… <NA> NA NA NA
2 32043rh " 22… "344… " 5… " … " … Illumin… NA 1 1
3 32043rh " 22… "344… " 5… " … " … Illumin… NA 1 1
4 32044rh " … " … "348… " … " 16… Illumin… NA 1 1
5 32044rh " … " … "348… " … " 16… Illumin… NA 1 1
6 32046rh " … " … "394… " … " 13… Illumin… NA 1 1
7 32046rh " … " … "394… " … " 13… Illumin… NA 1 1
8 32047rh " … " … "308… " … " 12… Illumin… NA 1 1
9 32047rh " … " … "308… " … " 12… Illumin… NA 1 1
10 32048rh " 16… "260… " 2… " … " … Illumin… NA 1 1
# ℹ 135 more rows
# ℹ 45 more variables: sequencingBatch <dbl>, libraryPrep <chr>,
# libraryPreparationMethod <lgl>, isStranded <lgl>, readStrandOrigin <lgl>,
# runType <chr>, readLength <dbl>, individualID <dbl>,
# specimenIdSource <chr>, organ <chr>, tissue <chr>, BrodmannArea <lgl>,
# sampleStatus <chr>, tissueWeight <lgl>, tissueVolume <lgl>,
# nucleicAcidSource <lgl>, cellType <lgl>, fastingState <lgl>, …
Challenge 1
Download the raw read counts and the metadata from the Jax.IU.Pitt_APOE4.TREM2.R47H study (syn22107627, syn23613784).
Solution to Challenge 1
counts_id <- "syn22107627" synGet(counts_id, downloadLocation = "../data/") metadata_id <- 'syn23613784' synGet(metadata_id, downloadLocation = "../data/")
Key Points
Use your Synapse login credentials to access the Portal.
Use Synapser package to download data from the Portal.
Differential Expression Analysis
Overview
Teaching: 40 min
Exercises: 10 minQuestions
What transcriptomic changes we observe in mouse models carrying AD-related mutations?
Objectives
Read in a count matrix and metadata.
Understand the data from AD mouse models
Format the data for differential analysis
Perform differential analysis using DESeq2.
Pathway enrichment of differentially expressed genes
Save data for next lessons
Author: Ravi Pandey, Jackson Laboratory
LOAD libraries
suppressPackageStartupMessages(library("DESeq2"))
suppressPackageStartupMessages(library("ggplot2"))
suppressPackageStartupMessages(library("AnnotationDbi"))
suppressPackageStartupMessages(library("org.Mm.eg.db"))
suppressPackageStartupMessages(library("GO.db"))
suppressPackageStartupMessages(library("EnhancedVolcano"))
suppressPackageStartupMessages(library(tidyverse))
suppressPackageStartupMessages(library(dplyr))
suppressPackageStartupMessages(library(clusterProfiler))
Reading Gene Expression Count matrix from Previous Lesson
In this lesson, we will use the raw counts matrix and metadata downloaded in the previous lesson and will perform differential expression analysis.
RNA-Seq data from 5xFAD mouse models
counts <- read.delim("../data/htseqcounts_5XFAD.txt", check.names = FALSE)
Reading Sample Metadata from Previous Lesson
covars <- readRDS("../data/covars_5XFAD.rds")
Let’s explore the data:
Let’s look at the top of the metadata.
head(covars)
individualID specimenID sex genotype timepoint
32043rh 32043 32043rh female 5XFAD_carrier 12 mo
32044rh 32044 32044rh male 5XFAD_noncarrier 12 mo
32046rh 32046 32046rh male 5XFAD_noncarrier 12 mo
32047rh 32047 32047rh male 5XFAD_noncarrier 12 mo
32049rh 32049 32049rh female 5XFAD_noncarrier 12 mo
32057rh 32057 32057rh female 5XFAD_noncarrier 12 mo
identify distinct groups using sample metadata
distinct(covars, sex, genotype, timepoint)
sex genotype timepoint
32043rh female 5XFAD_carrier 12 mo
32044rh male 5XFAD_noncarrier 12 mo
32049rh female 5XFAD_noncarrier 12 mo
46105rh female 5XFAD_noncarrier 6 mo
46108rh male 5XFAD_noncarrier 6 mo
46131rh female 5XFAD_noncarrier 4 mo
46877rh male 5XFAD_noncarrier 4 mo
46887rh female 5XFAD_carrier 4 mo
32053rh male 5XFAD_carrier 12 mo
46111rh female 5XFAD_carrier 6 mo
46865rh male 5XFAD_carrier 6 mo
46866rh male 5XFAD_carrier 4 mo
We’re going to explore the data further using a series of Challenges. You will be asked to look at the contents of some of the columns to see how the data are distributed.
Challenge 1
How many mice were used to produce this data?
Solution to Challenge 1
covars %>% group_by(sex,genotype,timepoint) %>% count() dplyr::count(metadata, sex, genotype,timepoint)
How many rows and columns are there in counts?
dim(counts)
[1] 55489 73
In the counts matrix, genes are in rows and samples are in columns. Let’s look at the first few rows.
head(counts,n=5)
gene_id 32043rh 32044rh 32046rh 32047rh 32048rh 32049rh 32050rh
1 ENSG00000080815 22554 0 0 0 16700 0 0
2 ENSG00000142192 344489 4 0 1 260935 6 8
3 ENSMUSG00000000001 5061 3483 3941 3088 2756 3067 2711
4 ENSMUSG00000000003 0 0 0 0 0 0 0
5 ENSMUSG00000000028 208 162 138 127 95 154 165
32052rh 32053rh 32057rh 32059rh 32061rh 32062rh 32065rh 32067rh 32068rh
1 19748 14023 0 17062 0 15986 10 0 18584
2 337456 206851 1 264748 0 252248 172 4 300398
3 3334 3841 4068 3306 4076 3732 3940 4238 3257
4 0 0 0 0 0 0 0 0 0
5 124 103 164 116 108 134 204 239 148
32070rh 32073rh 32074rh 32075rh 32078rh 32081rh 32088rh 32640rh 46105rh
1 1 0 0 22783 17029 16626 15573 12721 4
2 4 2 9 342655 280968 258597 243373 188818 19
3 3351 3449 4654 4844 3132 3334 3639 3355 4191
4 0 0 0 0 0 0 0 0 0
5 159 167 157 211 162 149 160 103 158
46106rh 46107rh 46108rh 46109rh 46110rh 46111rh 46112rh 46113rh 46115rh
1 0 0 0 0 0 17931 0 19087 0
2 0 0 1 5 1 293409 8 273704 1
3 3058 4265 3248 3638 3747 3971 3192 3805 3753
4 0 0 0 0 0 0 0 0 0
5 167 199 113 168 175 203 158 108 110
46121rh 46131rh 46132rh 46134rh 46138rh 46141rh 46142rh 46862rh 46863rh
1 0 0 12703 18833 0 18702 17666 0 14834
2 0 1 187975 285048 0 284499 250600 0 218494
3 4134 3059 3116 3853 3682 2844 3466 3442 3300
4 0 0 0 0 0 0 0 0 0
5 179 137 145 183 171 138 88 154 157
46865rh 46866rh 46867rh 46868rh 46871rh 46872rh 46873rh 46874rh 46875rh
1 10546 10830 10316 10638 15248 0 0 11608 11561
2 169516 152769 151732 190150 229063 6 1 165941 171303
3 3242 3872 3656 3739 3473 3154 5510 3657 4121
4 0 0 0 0 0 0 0 0 0
5 131 152 152 155 140 80 240 148 112
46876rh 46877rh 46878rh 46879rh 46881rh 46882rh 46883rh 46884rh 46885rh
1 0 0 12683 15613 0 14084 20753 0 0
2 0 2 183058 216122 0 199448 306081 0 5
3 3422 3829 3996 4324 2592 2606 4600 2913 3614
4 0 0 0 0 0 0 0 0 0
5 147 166 169 215 115 101 174 127 151
46886rh 46887rh 46888rh 46889rh 46890rh 46891rh 46892rh 46893rh 46895rh
1 16639 16072 0 16680 13367 0 25119 92 0
2 242543 258061 0 235530 196721 0 371037 1116 0
3 3294 3719 3899 4173 4008 3037 5967 3459 4262
4 0 0 0 0 0 0 0 0 0
5 139 128 210 127 156 116 260 161 189
46896rh 46897rh
1 15934 0
2 235343 6
3 3923 3486
4 0 0
5 179 117
As you can see, the gene ids are ENSEBL IDs. There is some risk that these may not be unique. Let’s check whether any of the gene symbols are duplicated. We will sum the number of duplicated gene symbols.
sum(duplicated(rownames(counts)))
[1] 0
The sum equals zero, so there are no duplicated gene symbols, which is good. Similarly, samples should be unique. Once again, let’s verify this:
sum(duplicated(colnames(counts)))
[1] 0
Formatting the count matrix
Now, as we see that gene_id is in first column of count matrix, but we will need only count data in matrix, so we will change the gene_id column to rownames.
Converting the gene_id as rownames of count matrix
counts <- counts %>% column_to_rownames(.,var="gene_id") %>% as.data.frame()
let’s confirm if change is done correctly
head(counts,n=5)
32043rh 32044rh 32046rh 32047rh 32048rh 32049rh 32050rh
ENSG00000080815 22554 0 0 0 16700 0 0
ENSG00000142192 344489 4 0 1 260935 6 8
ENSMUSG00000000001 5061 3483 3941 3088 2756 3067 2711
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 208 162 138 127 95 154 165
32052rh 32053rh 32057rh 32059rh 32061rh 32062rh 32065rh
ENSG00000080815 19748 14023 0 17062 0 15986 10
ENSG00000142192 337456 206851 1 264748 0 252248 172
ENSMUSG00000000001 3334 3841 4068 3306 4076 3732 3940
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 124 103 164 116 108 134 204
32067rh 32068rh 32070rh 32073rh 32074rh 32075rh 32078rh
ENSG00000080815 0 18584 1 0 0 22783 17029
ENSG00000142192 4 300398 4 2 9 342655 280968
ENSMUSG00000000001 4238 3257 3351 3449 4654 4844 3132
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 239 148 159 167 157 211 162
32081rh 32088rh 32640rh 46105rh 46106rh 46107rh 46108rh
ENSG00000080815 16626 15573 12721 4 0 0 0
ENSG00000142192 258597 243373 188818 19 0 0 1
ENSMUSG00000000001 3334 3639 3355 4191 3058 4265 3248
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 149 160 103 158 167 199 113
46109rh 46110rh 46111rh 46112rh 46113rh 46115rh 46121rh
ENSG00000080815 0 0 17931 0 19087 0 0
ENSG00000142192 5 1 293409 8 273704 1 0
ENSMUSG00000000001 3638 3747 3971 3192 3805 3753 4134
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 168 175 203 158 108 110 179
46131rh 46132rh 46134rh 46138rh 46141rh 46142rh 46862rh
ENSG00000080815 0 12703 18833 0 18702 17666 0
ENSG00000142192 1 187975 285048 0 284499 250600 0
ENSMUSG00000000001 3059 3116 3853 3682 2844 3466 3442
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 137 145 183 171 138 88 154
46863rh 46865rh 46866rh 46867rh 46868rh 46871rh 46872rh
ENSG00000080815 14834 10546 10830 10316 10638 15248 0
ENSG00000142192 218494 169516 152769 151732 190150 229063 6
ENSMUSG00000000001 3300 3242 3872 3656 3739 3473 3154
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 157 131 152 152 155 140 80
46873rh 46874rh 46875rh 46876rh 46877rh 46878rh 46879rh
ENSG00000080815 0 11608 11561 0 0 12683 15613
ENSG00000142192 1 165941 171303 0 2 183058 216122
ENSMUSG00000000001 5510 3657 4121 3422 3829 3996 4324
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 240 148 112 147 166 169 215
46881rh 46882rh 46883rh 46884rh 46885rh 46886rh 46887rh
ENSG00000080815 0 14084 20753 0 0 16639 16072
ENSG00000142192 0 199448 306081 0 5 242543 258061
ENSMUSG00000000001 2592 2606 4600 2913 3614 3294 3719
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 115 101 174 127 151 139 128
46888rh 46889rh 46890rh 46891rh 46892rh 46893rh 46895rh
ENSG00000080815 0 16680 13367 0 25119 92 0
ENSG00000142192 0 235530 196721 0 371037 1116 0
ENSMUSG00000000001 3899 4173 4008 3037 5967 3459 4262
ENSMUSG00000000003 0 0 0 0 0 0 0
ENSMUSG00000000028 210 127 156 116 260 161 189
46896rh 46897rh
ENSG00000080815 15934 0
ENSG00000142192 235343 6
ENSMUSG00000000001 3923 3486
ENSMUSG00000000003 0 0
ENSMUSG00000000028 179 117
As you can see from count table there are some genes that start with “ENSG” and others start with “ENSMUSG”. “ENSG” referes to human gene ENSEMBL id and “ENSMUSG” refer to mouse ENSEMBL id. Let’s check how many gene_ids are NOT from the mouse genome by searching for the string “MUS” (as in Mus musculus) in the rownames of count matrix
counts[,1:6] %>%
filter(!str_detect(rownames(.), "MUS"))
32043rh 32044rh 32046rh 32047rh 32048rh 32049rh
ENSG00000080815 22554 0 0 0 16700 0
ENSG00000142192 344489 4 0 1 260935 6
Ok, so we see there are two human genes in out count matrix. Why? What genes are they?
Briefly, 5xFAD mouse strain harbors two human transgenes APP (“ENSG00000142192”) and PSEN1 (“ENSG00000080815”) and inserted into exon 2 of the mouse Thy1 gene. To validate 5XFAD strain and capture expression of human transgene APP and PS1, a custom mouse genomic sequences was created and we quantified expression of human as well as mouse App (“ENSMUSG00000022892”) and Psen1 (“ENSMUSG00000019969”) genes by our MODEL-AD RNA-Seq pipeline.
Validation of 5xFAD mouse strain
#First we convert the dataframe to longer format and join our covariates by MouseID
count_tpose <- counts %>%
rownames_to_column(.,var="gene_id") %>%
filter(gene_id %in% c("ENSG00000080815","ENSMUSG00000019969","ENSG00000142192","ENSMUSG00000022892")) %>%
pivot_longer(.,cols = -"gene_id",names_to = "specimenID",values_to="counts") %>% as.data.frame() %>%
left_join(covars ,by="specimenID") %>% as.data.frame()
head(count_tpose)
gene_id specimenID counts individualID sex genotype
1 ENSG00000080815 32043rh 22554 32043 female 5XFAD_carrier
2 ENSG00000080815 32044rh 0 32044 male 5XFAD_noncarrier
3 ENSG00000080815 32046rh 0 32046 male 5XFAD_noncarrier
4 ENSG00000080815 32047rh 0 32047 male 5XFAD_noncarrier
5 ENSG00000080815 32048rh 16700 32048 female 5XFAD_carrier
6 ENSG00000080815 32049rh 0 32049 female 5XFAD_noncarrier
timepoint
1 12 mo
2 12 mo
3 12 mo
4 12 mo
5 12 mo
6 12 mo
#make the age column a factor and re-order the levels
count_tpose$timepoint <- factor(count_tpose$timepoint,levels=c("4 mo","6 mo","12 mo"))
# rename the gene id to gene symbol
count_tpose$gene_id[count_tpose$gene_id %in% "ENSG00000142192"] <- "Human APP"
count_tpose$gene_id[count_tpose$gene_id %in% "ENSG00000080815"] <- "Human PSEN1"
count_tpose$gene_id[count_tpose$gene_id %in% "ENSMUSG00000022892"] <- "Mouse App"
count_tpose$gene_id[count_tpose$gene_id %in% "ENSMUSG00000019969"] <- "Mouse Psen1"
#Create simple box plots showing normalized counts by genotype and time point, faceted by sex.
count_tpose %>%
ggplot(aes(x=timepoint, y=counts, color=genotype)) +
geom_boxplot() +
geom_point(position=position_jitterdodge()) +
facet_wrap(~sex+gene_id) +theme_bw()
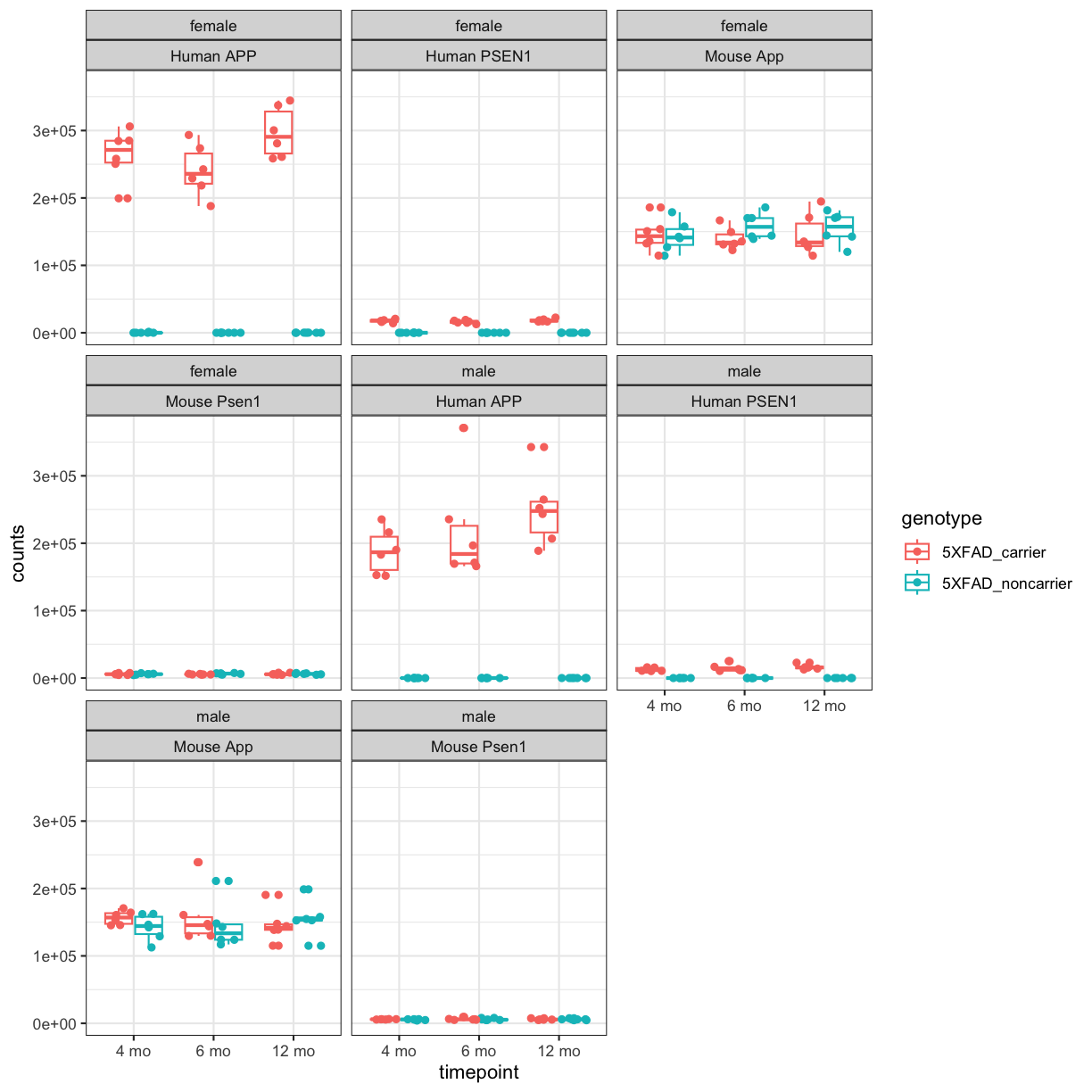
plot of chunk counts_boxplot
You will notice expression of Human APP is higher in 5XFAD carriers but lower in non-carriers. However mouse App expressed in both 5XFAD carrier and non-carrier.
We are going to sum the counts from both ortholgous genes (human APP and mouse App; human PSEN1 and mouse Psen1) and save summed expression as expression of mouse genes, respectively to match with gene names in control mice.
#merge mouse and human APP gene raw count
counts[rownames(counts) %in% "ENSMUSG00000022892",] <- counts[rownames(counts) %in% "ENSMUSG00000022892",] + counts[rownames(counts) %in% "ENSG00000142192",]
counts <- counts[!rownames(counts) %in% c("ENSG00000142192"),]
#merge mouse and human PS1 gene raw count
counts[rownames(counts) %in% "ENSMUSG00000019969",] <- counts[rownames(counts) %in% "ENSMUSG00000019969",] + counts[rownames(counts) %in% "ENSG00000080815",]
counts <- counts[!rownames(counts) %in% c("ENSG00000080815"),]
Let’s verify if expression of both human genes have been merged or not:
counts[,1:6] %>%
filter(!str_detect(rownames(.), "MUS"))
[1] 32043rh 32044rh 32046rh 32047rh 32048rh 32049rh
<0 rows> (or 0-length row.names)
What proportion of genes have zero counts in all samples?
gene_sums <- data.frame(gene_id = rownames(counts),
sums = Matrix::rowSums(counts))
sum(gene_sums$sums == 0)
[1] 9691
We can see that 9691 (17%) genes have no reads at all associated with them. In the next lesson, we will remove genes that have no counts in any samples.
Differential Analysis using DESeq2
Now, after exploring and formatting the data, We will look for differential expression between the control and 5xFAD mice at different ages for both sexes. The differentially expressed genes (DEGs) can inform our understanding of how the 5XFAD mutation affect the biological processes.
DESeq2 analysis consist of multiple steps. We are going to briefly understand some of the important steps using subset of data and then we will perform differential analysis on whole dataset. For detailed analysis see the DESeq2 tutorial.
First, order the data (so counts and metadata match) and save in another variable
rawdata <- counts[,sort(colnames(counts))]
metadata <- covars[sort(rownames(covars)),]
subset the counts matrix and sample metadata to include only 12 month old male mice. You can amend the code to compare wild type and 5XFAD mice from either sex, at any time point.
meta.12M.Male <- metadata[(metadata$sex=="male" & metadata$timepoint=='12 mo'),]
meta.12M.Male
individualID specimenID sex genotype timepoint
32044rh 32044 32044rh male 5XFAD_noncarrier 12 mo
32046rh 32046 32046rh male 5XFAD_noncarrier 12 mo
32047rh 32047 32047rh male 5XFAD_noncarrier 12 mo
32053rh 32053 32053rh male 5XFAD_carrier 12 mo
32059rh 32059 32059rh male 5XFAD_carrier 12 mo
32061rh 32061 32061rh male 5XFAD_noncarrier 12 mo
32062rh 32062 32062rh male 5XFAD_carrier 12 mo
32073rh 32073 32073rh male 5XFAD_noncarrier 12 mo
32074rh 32074 32074rh male 5XFAD_noncarrier 12 mo
32075rh 32075 32075rh male 5XFAD_carrier 12 mo
32088rh 32088 32088rh male 5XFAD_carrier 12 mo
32640rh 32640 32640rh male 5XFAD_carrier 12 mo
dat <- as.matrix(rawdata[,colnames(rawdata) %in% rownames(meta.12M.Male)])
colnames(dat)
[1] "32044rh" "32046rh" "32047rh" "32053rh" "32059rh" "32061rh" "32062rh"
[8] "32073rh" "32074rh" "32075rh" "32088rh" "32640rh"
rownames(meta.12M.Male)
[1] "32044rh" "32046rh" "32047rh" "32053rh" "32059rh" "32061rh" "32062rh"
[8] "32073rh" "32074rh" "32075rh" "32088rh" "32640rh"
match(colnames(dat),rownames(meta.12M.Male))
[1] 1 2 3 4 5 6 7 8 9 10 11 12
Next, we build the DESeqDataSet using the following function:
ddsHTSeq <- DESeqDataSetFromMatrix(countData=dat,
colData=meta.12M.Male,
design = ~genotype)
Warning in DESeqDataSet(se, design = design, ignoreRank): some variables in
design formula are characters, converting to factors
ddsHTSeq
class: DESeqDataSet
dim: 55487 12
metadata(1): version
assays(1): counts
rownames(55487): ENSMUSG00000000001 ENSMUSG00000000003 ...
ENSMUSG00000118487 ENSMUSG00000118488
rowData names(0):
colnames(12): 32044rh 32046rh ... 32088rh 32640rh
colData names(5): individualID specimenID sex genotype timepoint
Pre-filtering
While it is not necessary to pre-filter low count genes before running the DESeq2 functions, there are two reasons which make pre-filtering useful: by removing rows in which there are very few reads, we reduce the memory size of the dds data object, and we increase the speed of the transformation and testing functions within DESeq2. It can also improve visualizations, as features with no information for differential expression are not plotted.
Here we perform a minimal pre-filtering to keep only rows that have at least 10 reads total.
ddsHTSeq <- ddsHTSeq[rowSums(counts(ddsHTSeq)) >= 10,]
ddsHTSeq
class: DESeqDataSet
dim: 33059 12
metadata(1): version
assays(1): counts
rownames(33059): ENSMUSG00000000001 ENSMUSG00000000028 ...
ENSMUSG00000118486 ENSMUSG00000118487
rowData names(0):
colnames(12): 32044rh 32046rh ... 32088rh 32640rh
colData names(5): individualID specimenID sex genotype timepoint
Reference level
By default, R will choose a reference level for factors based on alphabetical order. Then, if you never tell the DESeq2 functions which level you want to compare against (e.g. which level represents the control group), the comparisons will be based on the alphabetical order of the levels.
specifying the reference-level to 5XFAD_noncarrier:
ddsHTSeq$genotype <- relevel(ddsHTSeq$genotype,ref="5XFAD_noncarrier")
Run the standard differential expression analysis steps that is wrapped into a single function, DESeq.
dds <- DESeq(ddsHTSeq,parallel = TRUE)
Results tables are generated using the function results, which extracts a results table with log2 fold changes, p values and adjusted p values. By default the argument alpha is set to 0.1. If the adjusted p value cutoff will be a value other than 0.1, alpha should be set to that value:
res <- results(dds,alpha=0.05) # setting 0.05 as significant threshold
res
log2 fold change (MLE): genotype 5XFAD carrier vs 5XFAD noncarrier
Wald test p-value: genotype 5XFAD carrier vs 5XFAD noncarrier
DataFrame with 33059 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue
<numeric> <numeric> <numeric> <numeric> <numeric>
ENSMUSG00000000001 3737.9009 0.0148125 0.0466948 0.317219 0.7510777
ENSMUSG00000000028 138.5635 -0.0712500 0.1550130 -0.459639 0.6457754
ENSMUSG00000000031 29.2983 0.6705922 0.3563441 1.881867 0.0598541
ENSMUSG00000000037 123.6482 -0.2184054 0.1554362 -1.405113 0.1599876
ENSMUSG00000000049 15.1733 0.3657555 0.3924376 0.932009 0.3513317
... ... ... ... ... ...
ENSMUSG00000118473 1.18647 -0.377971 1.531586 -0.246784 0.805075
ENSMUSG00000118477 59.10359 -0.144081 0.226690 -0.635586 0.525046
ENSMUSG00000118479 24.64566 -0.181992 0.341445 -0.533006 0.594029
ENSMUSG00000118486 1.92048 0.199838 1.253875 0.159376 0.873372
ENSMUSG00000118487 65.78311 -0.191362 0.218593 -0.875427 0.381342
padj
<numeric>
ENSMUSG00000000001 0.943421
ENSMUSG00000000028 0.913991
ENSMUSG00000000031 0.352346
ENSMUSG00000000037 0.566360
ENSMUSG00000000049 0.765640
... ...
ENSMUSG00000118473 NA
ENSMUSG00000118477 0.863565
ENSMUSG00000118479 0.893356
ENSMUSG00000118486 NA
ENSMUSG00000118487 0.785845
We can order our results table by the smallest p value:
resOrdered <- res[order(res$pvalue),]
head(resOrdered,n=10)
log2 fold change (MLE): genotype 5XFAD carrier vs 5XFAD noncarrier
Wald test p-value: genotype 5XFAD carrier vs 5XFAD noncarrier
DataFrame with 10 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue
<numeric> <numeric> <numeric> <numeric> <numeric>
ENSMUSG00000019969 13860.942 1.90740 0.0432685 44.0828 0.00000e+00
ENSMUSG00000030579 2367.096 2.61215 0.0749325 34.8600 2.99877e-266
ENSMUSG00000046805 7073.296 2.12247 0.0635035 33.4229 6.38260e-245
ENSMUSG00000032011 80423.476 1.36195 0.0424006 32.1210 2.24185e-226
ENSMUSG00000022892 271265.838 1.36140 0.0434167 31.3567 7.88666e-216
ENSMUSG00000038642 10323.969 1.69717 0.0549488 30.8864 1.81838e-209
ENSMUSG00000023992 2333.227 2.62290 0.0882818 29.7105 5.61657e-194
ENSMUSG00000079293 761.313 5.12514 0.1738382 29.4822 4.86644e-191
ENSMUSG00000040552 617.149 2.22726 0.0781799 28.4889 1.60622e-178
ENSMUSG00000069516 2604.926 2.34471 0.0847390 27.6697 1.61585e-168
padj
<numeric>
ENSMUSG00000019969 0.00000e+00
ENSMUSG00000030579 3.60828e-262
ENSMUSG00000046805 5.11991e-241
ENSMUSG00000032011 1.34876e-222
ENSMUSG00000022892 3.79585e-212
ENSMUSG00000038642 7.29321e-206
ENSMUSG00000023992 1.93090e-190
ENSMUSG00000079293 1.46389e-187
ENSMUSG00000040552 4.29486e-175
ENSMUSG00000069516 3.88853e-165
we can summarize some basic tallies using the summary function.
summary(res)
out of 33059 with nonzero total read count
adjusted p-value < 0.05
LFC > 0 (up) : 1098, 3.3%
LFC < 0 (down) : 505, 1.5%
outliers [1] : 33, 0.1%
low counts [2] : 8961, 27%
(mean count < 8)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
How many adjusted p-values were less than 0.05?
sum(res$padj < 0.05, na.rm=TRUE)
[1] 1603
Challenge 2
How many adjusted p-values were less than 0.1?
Solution to Challenge 2
sum(res$padj < 0.1, na.rm=TRUE)
Function to convert ensembleIDs to common gene names
We’ll use a package to translate mouse ENSEMBL IDS to gene names. Run this function and they will be called up when assembling results from the differential expression analysis
map_function.df <- function(x, inputtype, outputtype) {
mapIds(
org.Mm.eg.db,
keys = row.names(x),
column = outputtype,
keytype = inputtype,
multiVals = "first"
)
}
Generating Result table
All_res <- as.data.frame(res) %>%
mutate(symbol = map_function.df(res,"ENSEMBL","SYMBOL")) %>% ##run map_function to add symbol of gene corresponding to ENSEBL ID
mutate(EntrezGene = map_function.df(res,"ENSEMBL","ENTREZID")) %>% ##run map_function to add Entrez ID of gene corresponding to ENSEBL ID
dplyr::select("symbol", "EntrezGene","baseMean", "log2FoldChange", "lfcSE", "stat", "pvalue", "padj")
head(All_res)
symbol EntrezGene baseMean log2FoldChange lfcSE
ENSMUSG00000000001 Gnai3 14679 3737.90089 0.01481247 0.04669482
ENSMUSG00000000028 Cdc45 12544 138.56354 -0.07125004 0.15501305
ENSMUSG00000000031 H19 14955 29.29832 0.67059217 0.35634414
ENSMUSG00000000037 Scml2 107815 123.64823 -0.21840544 0.15543617
ENSMUSG00000000049 Apoh 11818 15.17325 0.36575554 0.39243763
ENSMUSG00000000056 Narf 67608 5017.30216 -0.06713961 0.04466809
stat pvalue padj
ENSMUSG00000000001 0.3172187 0.75107767 0.9434210
ENSMUSG00000000028 -0.4596390 0.64577536 0.9139907
ENSMUSG00000000031 1.8818667 0.05985412 0.3523457
ENSMUSG00000000037 -1.4051134 0.15998757 0.5663600
ENSMUSG00000000049 0.9320093 0.35133170 0.7656399
ENSMUSG00000000056 -1.5030778 0.13281898 0.5203330
Extracting genes that are significantly expressed
dseq_res <- subset(All_res[order(All_res$padj), ], padj < 0.05)
Wow! We have a lot of genes with apparently very strong statistically significant differences between the control and 5xFAD carrier.
dim(dseq_res)
[1] 1603 8
head(dseq_res)
symbol EntrezGene baseMean log2FoldChange lfcSE
ENSMUSG00000019969 Psen1 19164 13860.942 1.907397 0.04326854
ENSMUSG00000030579 Tyrobp 22177 2367.096 2.612152 0.07493255
ENSMUSG00000046805 Mpeg1 17476 7073.296 2.122467 0.06350346
ENSMUSG00000032011 Thy1 21838 80423.476 1.361953 0.04240065
ENSMUSG00000022892 App 11820 271265.838 1.361405 0.04341673
ENSMUSG00000038642 Ctss 13040 10323.969 1.697172 0.05494882
stat pvalue padj
ENSMUSG00000019969 44.08278 0.000000e+00 0.000000e+00
ENSMUSG00000030579 34.86005 2.998775e-266 3.608276e-262
ENSMUSG00000046805 33.42286 6.382596e-245 5.119906e-241
ENSMUSG00000032011 32.12104 2.241854e-226 1.348756e-222
ENSMUSG00000022892 31.35669 7.886662e-216 3.795850e-212
ENSMUSG00000038642 30.88641 1.818378e-209 7.293211e-206
Exploring and exporting results
Exporting results to CSV files
we can save results file into a csv file like this:
write.csv(All_res,file="../results/All_5xFAD_12months_male.csv")
write.csv(dseq_res,file="../results/DEG_5xFAD_12months_male.csv")
Volcano plot
We can visualize the differential expression results using Volcano plot function from EnhancedVolcano package. For the most basic volcano plot, only a single data-frame, data-matrix, or tibble of test results is required, containing point labels, log2FC, and adjusted or unadjusted P values. The default cut-off for log2FC is >|2|; the default cut-off for P value is 10e-6.
EnhancedVolcano(All_res,
lab = (All_res$symbol),
x = 'log2FoldChange',
y = 'padj',legendPosition = 'none',
title = 'Volcano plot:Differential Expression Results',
subtitle = '',
FCcutoff = 0.1,
pCutoff = 0.05,
xlim = c(-3, 6))
Warning: One or more p-values is 0. Converting to 10^-1 * current lowest
non-zero p-value...
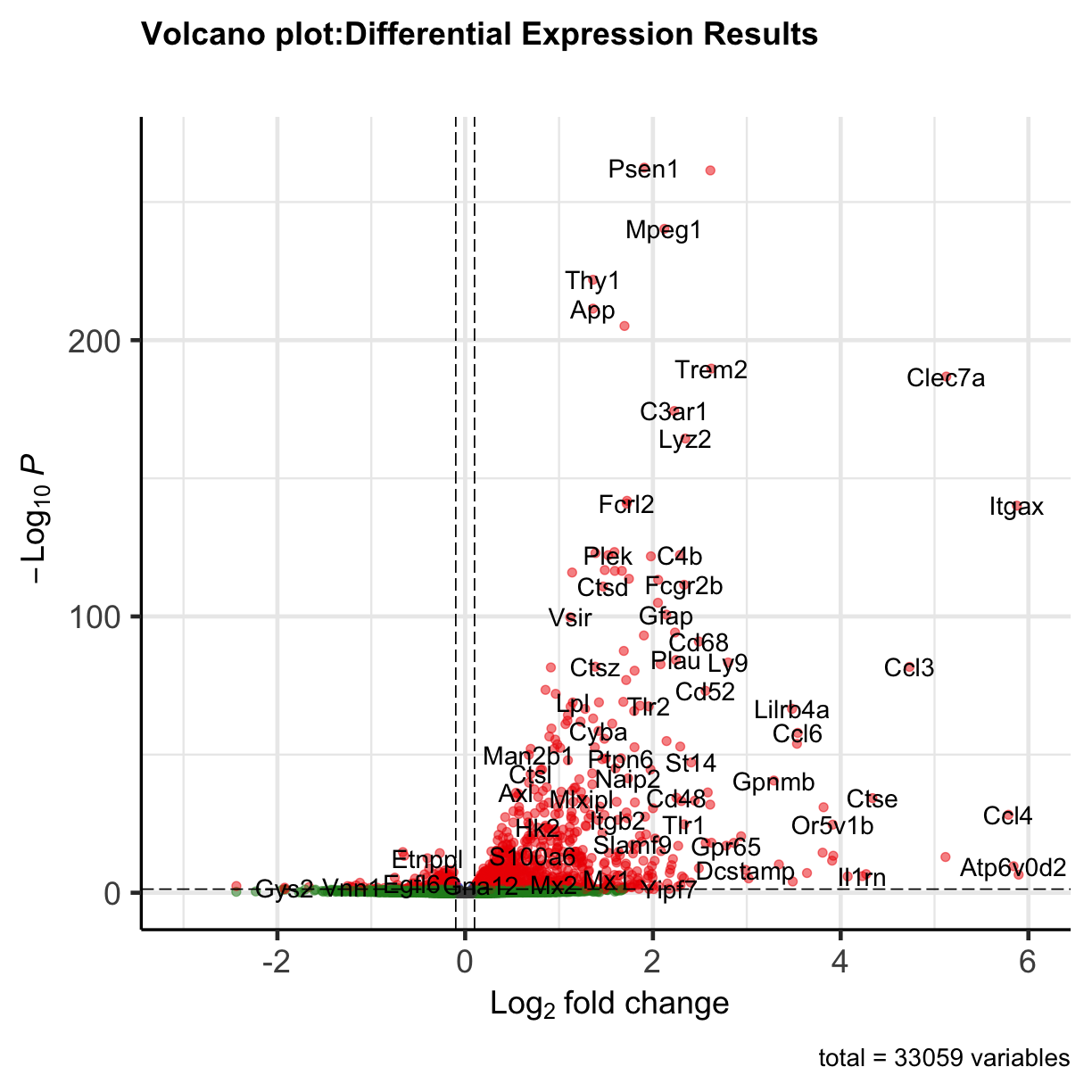
plot of chunk volcanoplot
You can see that some top significantly expressed are immune/inflammation-related genes such as Ctsd, C4b, Csf1 etc. These genes are upregulated in the 5XFAD strain.
Principal component plot of the samples
ddsHTSeq <- DESeqDataSetFromMatrix(countData=as.matrix(rawdata), colData=metadata, design= ~ genotype)
Warning in DESeqDataSet(se, design = design, ignoreRank): some variables in
design formula are characters, converting to factors
ddsHTSeq <- ddsHTSeq[rowSums(counts(ddsHTSeq)>1) >= 10, ]
dds <- DESeq(ddsHTSeq,parallel = TRUE)
estimating size factors
estimating dispersions
gene-wise dispersion estimates: 8 workers
mean-dispersion relationship
final dispersion estimates, fitting model and testing: 8 workers
-- replacing outliers and refitting for 42 genes
-- DESeq argument 'minReplicatesForReplace' = 7
-- original counts are preserved in counts(dds)
estimating dispersions
fitting model and testing
vsd <- varianceStabilizingTransformation(dds, blind=FALSE)
plotPCA(vsd, intgroup=c("genotype", "sex","timepoint"))
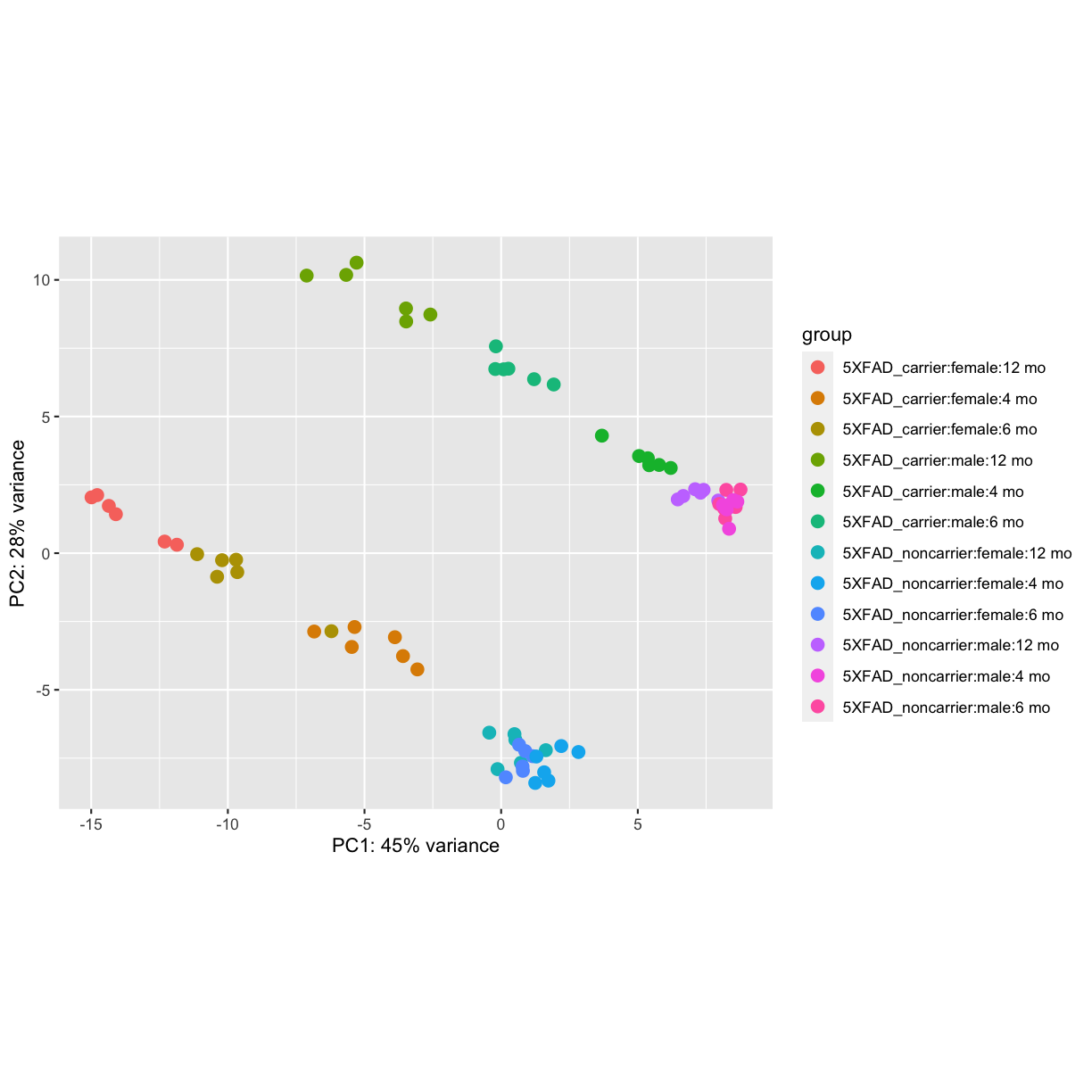
plot of chunk PCA
It is also possible to customize the PCA plot using the ggplot function.
pcaData <- plotPCA(vsd, intgroup=c("genotype", "sex","timepoint"), returnData=TRUE)
percentVar <- round(100 * attr(pcaData, "percentVar"))
ggplot(pcaData, aes(PC1, PC2,color=genotype, shape=sex)) +
geom_point(size=3) +
geom_text(aes(label=timepoint),hjust=0.5, vjust=2,size =3.5) +
labs(x= paste0("PC1: ",percentVar[1],"% variance"), y= paste0("PC2: ",percentVar[2],"% variance"))
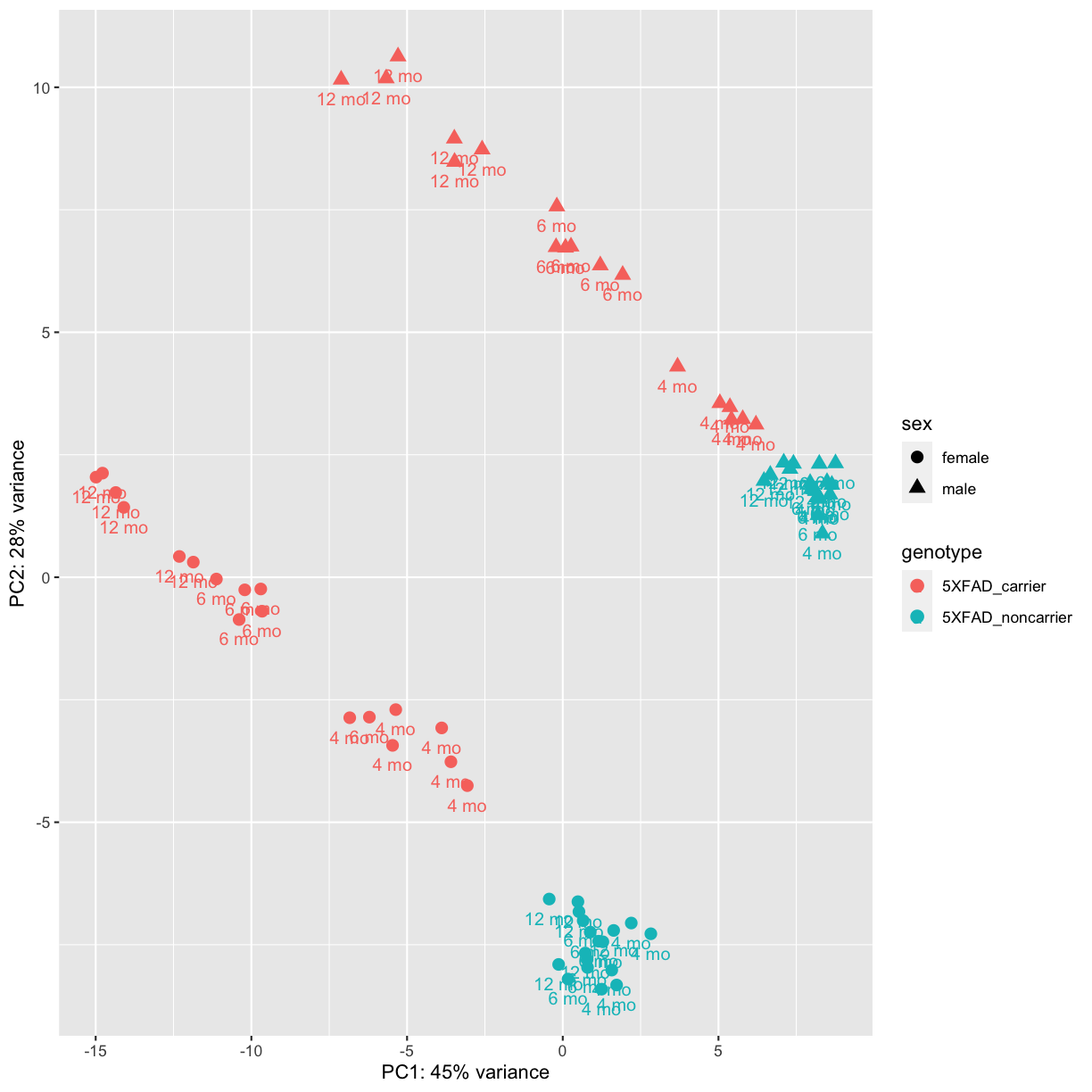
plot of chunk PCA2
PCA identified genotype and sex being a major source of variation in between 5XFAD and WT mice. Female and male samples clustered distinctly at all ages, suggesting the presence of sex-biased molecular changes in animals.
Function for Differential analysis using DESeq2
Finally, we can built a function for differential analysis that consist of all above discussed steps. It will require to input sorted raw count matrix, sample metadata and define the reference group.
DEG <- function(rawdata,meta,
include.batch = FALSE,
ref = ref) {
dseq_res <- data.frame()
All_res <- data.frame()
if (include.batch) {
cat("Including batch as covariate\n")
design_formula <- ~ Batch + genotype
}
else{
design_formula <- ~ genotype
}
dat2 <- as.matrix(rawdata[, colnames(rawdata) %in% rownames(meta)])
ddsHTSeq <-
DESeqDataSetFromMatrix(countData = dat2,
colData = meta,
design = design_formula)
ddsHTSeq <- ddsHTSeq[rowSums(counts(ddsHTSeq)) >= 10, ]
ddsHTSeq$genotype <- relevel(ddsHTSeq$genotype, ref = ref)
dds <- DESeq(ddsHTSeq, parallel = TRUE)
res <- results(dds, alpha = 0.05)
#summary(res)
res$symbol <- map_function.df(res,"ENSEMBL","SYMBOL")
res$EntrezGene <- map_function.df(res,"ENSEMBL","ENTREZID")
All_res <<- as.data.frame(res[, c("symbol", "EntrezGene","baseMean", "log2FoldChange", "lfcSE", "stat", "pvalue", "padj")])
}
Let’s use this function to analyze all groups present in our data.
Differential Analysis of all groups
First, we add a Group column to our metadata table that will combine all variable of interest for that sample.
metadata$Group <- paste0(metadata$genotype,"-",metadata$sex,"-",metadata$timepoint)
unique(metadata$Group)
[1] "5XFAD_carrier-female-12 mo" "5XFAD_noncarrier-male-12 mo"
[3] "5XFAD_noncarrier-female-12 mo" "5XFAD_carrier-male-12 mo"
[5] "5XFAD_noncarrier-female-6 mo" "5XFAD_noncarrier-male-6 mo"
[7] "5XFAD_carrier-female-6 mo" "5XFAD_noncarrier-female-4 mo"
[9] "5XFAD_carrier-female-4 mo" "5XFAD_carrier-male-6 mo"
[11] "5XFAD_carrier-male-4 mo" "5XFAD_noncarrier-male-4 mo"
Next, we create a comparison table that has all cases and controls that we would like to compare with each other. Here I have made comparison group for age and sex-matched 5xFAD carriers vs 5xFAD_noncarriers:
comparisons <- data.frame(control=c("5XFAD_noncarrier-male-4 mo", "5XFAD_noncarrier-female-4 mo", "5XFAD_noncarrier-male-6 mo",
"5XFAD_noncarrier-female-6 mo","5XFAD_noncarrier-male-12 mo", "5XFAD_noncarrier-female-12 mo"),
case=c("5XFAD_carrier-male-4 mo", "5XFAD_carrier-female-4 mo", "5XFAD_carrier-male-6 mo",
"5XFAD_carrier-female-6 mo","5XFAD_carrier-male-12 mo", "5XFAD_carrier-female-12 mo")
)
comparisons
control case
1 5XFAD_noncarrier-male-4 mo 5XFAD_carrier-male-4 mo
2 5XFAD_noncarrier-female-4 mo 5XFAD_carrier-female-4 mo
3 5XFAD_noncarrier-male-6 mo 5XFAD_carrier-male-6 mo
4 5XFAD_noncarrier-female-6 mo 5XFAD_carrier-female-6 mo
5 5XFAD_noncarrier-male-12 mo 5XFAD_carrier-male-12 mo
6 5XFAD_noncarrier-female-12 mo 5XFAD_carrier-female-12 mo
Finally, we implement our DEG function on each comparison and store the result table in a list and data frame:
# initiate an empty list and data frame to save results
DE_5xFAD.list <- list()
DE_5xFAD.df <- data.frame()
for (i in 1:nrow(comparisons))
{
meta <- metadata[metadata$Group %in% comparisons[i,],]
DEG(rawdata,meta,ref = "5XFAD_noncarrier")
#append results in data frame
DE_5xFAD.df <- rbind(DE_5xFAD.df,All_res %>% mutate(model="5xFAD",sex=unique(meta$sex),age=unique(meta$timepoint)))
#append results in list
DE_5xFAD.list[[i]] <- All_res
names(DE_5xFAD.list)[i] <- paste0(comparisons[i,2])
}
Let’s explore the result stored in our list:
names(DE_5xFAD.list)
[1] "5XFAD_carrier-male-4 mo" "5XFAD_carrier-female-4 mo"
[3] "5XFAD_carrier-male-6 mo" "5XFAD_carrier-female-6 mo"
[5] "5XFAD_carrier-male-12 mo" "5XFAD_carrier-female-12 mo"
We can easily extract result table for any group of interest by using $ and name of group. Let’s check top few rows from 5XFAD_carrier-male-4 mo group:
head(DE_5xFAD.list$`5XFAD_carrier-male-4 mo`)
symbol EntrezGene baseMean log2FoldChange lfcSE
ENSMUSG00000000001 Gnai3 14679 3707.53159 -0.023085865 0.0381646
ENSMUSG00000000028 Cdc45 12544 159.76225 -0.009444949 0.1322609
ENSMUSG00000000031 H19 14955 35.96987 0.453401555 0.2785245
ENSMUSG00000000037 Scml2 107815 126.82414 0.089394561 0.1377406
ENSMUSG00000000049 Apoh 11818 19.99721 0.115325837 0.3154875
ENSMUSG00000000056 Narf 67608 5344.21741 -0.100413295 0.0381182
stat pvalue padj
ENSMUSG00000000001 -0.60490264 0.545243691 0.9999518
ENSMUSG00000000028 -0.07141148 0.943070275 0.9999518
ENSMUSG00000000031 1.62786960 0.103552539 0.9999518
ENSMUSG00000000037 0.64900659 0.516334116 0.9999518
ENSMUSG00000000049 0.36554806 0.714702337 0.9999518
ENSMUSG00000000056 -2.63426085 0.008432068 0.5696800
Let’s check the result stored as dataframe:
head(DE_5xFAD.df)
symbol EntrezGene baseMean log2FoldChange lfcSE
ENSMUSG00000000001 Gnai3 14679 3707.53159 -0.023085865 0.0381646
ENSMUSG00000000028 Cdc45 12544 159.76225 -0.009444949 0.1322609
ENSMUSG00000000031 H19 14955 35.96987 0.453401555 0.2785245
ENSMUSG00000000037 Scml2 107815 126.82414 0.089394561 0.1377406
ENSMUSG00000000049 Apoh 11818 19.99721 0.115325837 0.3154875
ENSMUSG00000000056 Narf 67608 5344.21741 -0.100413295 0.0381182
stat pvalue padj model sex age
ENSMUSG00000000001 -0.60490264 0.545243691 0.9999518 5xFAD male 4 mo
ENSMUSG00000000028 -0.07141148 0.943070275 0.9999518 5xFAD male 4 mo
ENSMUSG00000000031 1.62786960 0.103552539 0.9999518 5xFAD male 4 mo
ENSMUSG00000000037 0.64900659 0.516334116 0.9999518 5xFAD male 4 mo
ENSMUSG00000000049 0.36554806 0.714702337 0.9999518 5xFAD male 4 mo
ENSMUSG00000000056 -2.63426085 0.008432068 0.5696800 5xFAD male 4 mo
Check if result is present for all ages:
unique((DE_5xFAD.df$age))
[1] "4 mo" "6 mo" "12 mo"
Check if result is present for both sexes:
unique((DE_5xFAD.df$sex))
[1] "male" "female"
Check number of genes in each group:
count(DE_5xFAD.df,model,sex,age)
model sex age n
1 5xFAD female 12 mo 33120
2 5xFAD female 4 mo 32930
3 5xFAD female 6 mo 33249
4 5xFAD male 12 mo 33059
5 5xFAD male 4 mo 33119
6 5xFAD male 6 mo 33375
Check number of genes significantly differentially expressed in all cases compared to age and sex-matched controls:
degs.up <- map(DE_5xFAD.list, ~length(which(.x$padj<0.05 & .x$log2FoldChange>0)))
degs.down <- map(DE_5xFAD.list, ~length(which(.x$padj<0.05 & .x$log2FoldChange<0)))
deg <- data.frame(Cases=names(degs.up), Up_DEGs.pval.05=as.vector(unlist(degs.up)),Down_DEGs.pval.05=as.vector(unlist(degs.down)))
knitr::kable(deg)
| Cases | Up_DEGs.pval.05 | Down_DEGs.pval.05 |
|---|---|---|
| 5XFAD_carrier-male-4 mo | 86 | 11 |
| 5XFAD_carrier-female-4 mo | 522 | 90 |
| 5XFAD_carrier-male-6 mo | 714 | 488 |
| 5XFAD_carrier-female-6 mo | 1081 | 409 |
| 5XFAD_carrier-male-12 mo | 1098 | 505 |
| 5XFAD_carrier-female-12 mo | 1494 | 1023 |
Interestingly, in females more genes are differentially expressed at 4 months and with age more genes are differentially expressed. Male mice is catching up female mice at later time-point.
Pathway Enrichment
We may wish to look for enrichment of biological pathways in a list of differentially expressed genes. Here we will test for enrichment of KEGG pathways using using enrichKEGG function in clusterProfiler package.
dat <- list(FAD_M_4=subset(DE_5xFAD.list$`5XFAD_carrier-male-4 mo`[order(DE_5xFAD.list$`5XFAD_carrier-male-4 mo`$padj), ], padj < 0.05) %>% pull(EntrezGene),
FAD_F_4=subset(DE_5xFAD.list$`5XFAD_carrier-female-4 mo`[order(DE_5xFAD.list$`5XFAD_carrier-female-4 mo`$padj), ], padj < 0.05) %>% pull(EntrezGene))
## perform enrichment analysis
enrich_pathway <- compareCluster(dat,
fun = "enrichKEGG",
pvalueCutoff = 0.05,
organism = "mmu"
)
enrich_pathway@compareClusterResult$Description <- gsub(" - Mus musculus \\(house mouse)","",enrich_pathway@compareClusterResult$Description)
Let’s plot top enriched functions using dotplot function of clusterProfiler package.
clusterProfiler::dotplot(enrich_pathway,showCategory=10,font.size = 14,title="Enriched KEGG Pathways")
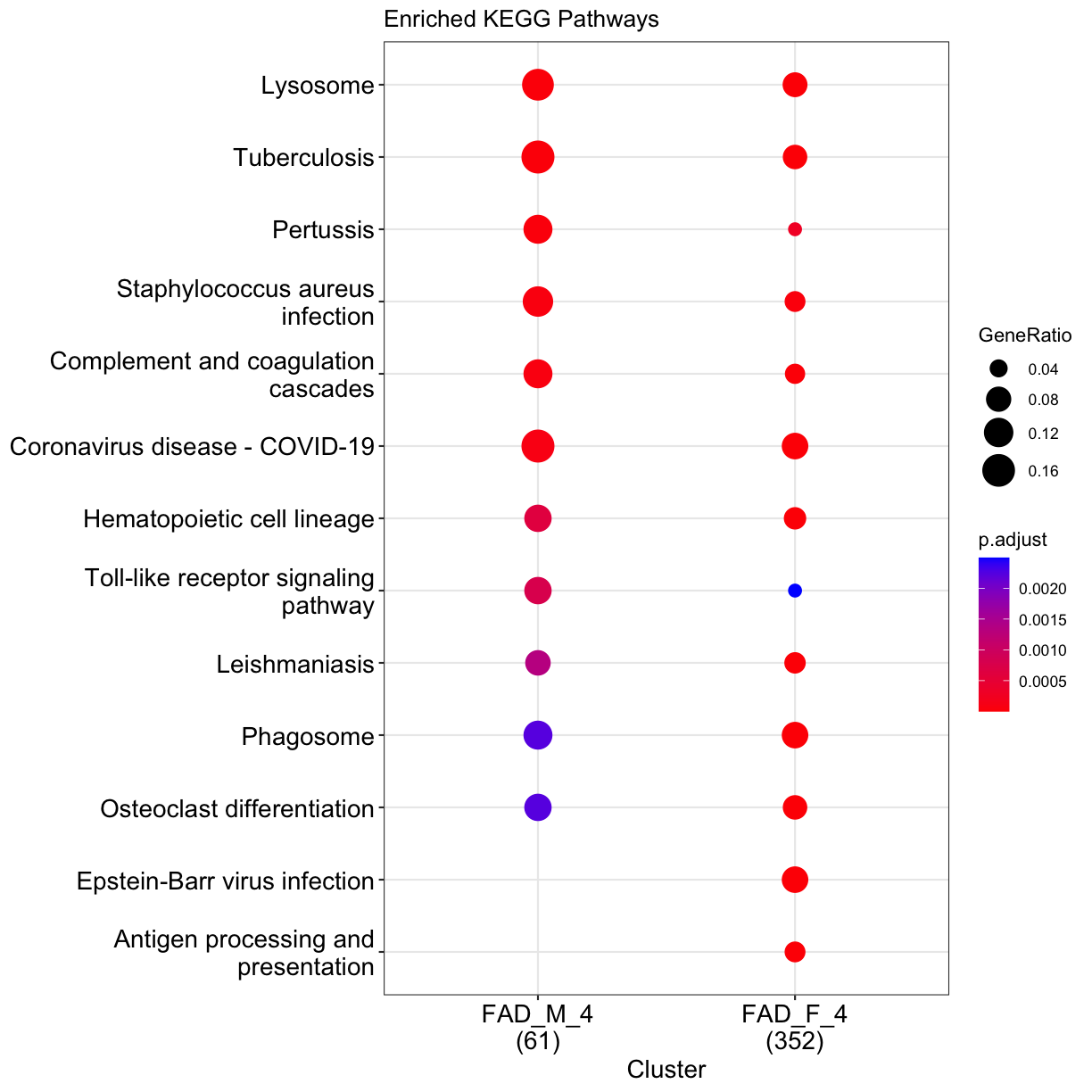
plot of chunk pathways
What does this plot infer?
Save Data for Next Lesson
We will use the results data in the next lesson. Save it now and we will load it at the beginning of the next lesson. We will use R’s save command to save the objects in compressed, binary format. The save command is useful when you want to save multiple objects in one file.
save(DE_5xFAD.df,DE_5xFAD.list,file="../results/DEAnalysis_5XFAD.Rdata")
Challenge 3
Draw volcano plot for 6 months old female 5xFAD_carrier ?
Solution to Challenge 3
EnhancedVolcano(DE_5xFAD.list$`5XFAD_carrier-female-6 mo, lab = (DE_5xFAD.list$`5XFAD_carrier-female-6 mo`$symbol), x = 'log2FoldChange', y = 'padj',legendPosition = 'none', title = 'Volcano plot:Differential Expression Results', subtitle = '', FCcutoff = 0.1, pCutoff = 0.05, xlim = c(-5, 5))
Loading RNA-Seq data from LOAD1 (APOE4*Trem2 Mouse Model)
There is another RNA-Seq data from LOAD1 mouse cohort. This cohort has three distinct mice strains: *APOE4: humanized Apoe knock-in strain, mouse Apoe allele was replaced by human APOE gene sequence; *Trem2.R47H:knock out mutant of the Trem2, R47H point mutation with two silent mutation and *APOE4.Trem2.R47H (LOAD1): double mutant strain carries humanized APOE (isoform 4) knock in mutation and R47H point mutation of the Trem2 gene.
Let’s read the count data and sample metadata as we did for 5XFAD samples.
counts <- read.delim("../data/htseqcounts_APTR.txt", check.names = FALSE)
ind_meta <- read.csv("../data/metadata_RNASEQ_ALLJAX_402Samples.csv")
Let’s look at the first few rows.
head(ind_meta)
Mouse.ID Sex Genotype Age Batch
1 123 M C57BL/6J 4 1
2 181 F C57BL/6J 4 1
3 191 M C57BL/6J 4 1
4 193 M C57BL/6J 4 1
5 221 F C57BL/6J 4 1
6 225 F C57BL/6J 4 1
Let’s quickly explore the sample metadata:
dim(ind_meta)
[1] 402 5
How many strains are in this sample metadata?
table(ind_meta$Genotype)
5XFAD Apoe4 APOE4 APOE4Trem2 ApoeKO APP Bin1
36 6 57 59 6 26 6
C57BL/6J Cd2ap Clu Trem2
137 6 6 57
How many samples are in each genotype for each sex and age group?
ind_meta %>% group_by(Sex,Genotype,Age) %>% count()
# A tibble: 51 × 4
# Groups: Sex, Genotype, Age [51]
Sex Genotype Age n
<chr> <chr> <int> <int>
1 F 5XFAD 4 6
2 F 5XFAD 6 6
3 F 5XFAD 12 6
4 F APOE4 4 12
5 F APOE4 8 6
6 F APOE4 12 5
7 F APOE4 24 6
8 F APOE4Trem2 4 12
9 F APOE4Trem2 8 6
10 F APOE4Trem2 12 5
# ℹ 41 more rows
We notice that there is a column Batch in sample metadata.
Let’s see how many samples are in each batch.
table(ind_meta$Batch)
1 2 3 4 5 6
140 94 72 36 48 12
So, total 5 batches. In bulk RNA-Seq experiments, it is usually vital that we apply a correction for samples profiled in different batches. Due to tim-constraint we do not want to do it in this lesson. So, we will use samples from only one batch in this lesson.
Let’s check which genotype are in which batch?
table(ind_meta$Batch,ind_meta$Genotype)
5XFAD Apoe4 APOE4 APOE4Trem2 ApoeKO APP Bin1 C57BL/6J Cd2ap Clu Trem2
1 0 0 33 35 0 0 0 36 0 0 36
2 0 0 24 24 0 0 0 25 0 0 21
3 36 0 0 0 0 0 0 36 0 0 0
4 0 6 0 0 6 0 6 6 6 6 0
5 0 0 0 0 0 16 0 32 0 0 0
6 0 0 0 0 0 10 0 2 0 0 0
We are going to use samples from Batch 1 as it has more samples and contain most of our LOAD1 cohort samples that we are interested.
Subset sample metadata for batch 1 only and Convert Mouse ID column to rownames.
covar <- ind_meta %>% filter(Batch==1) %>% remove_rownames() %>% column_to_rownames(.,var="Mouse.ID")
How many rows and columns are there in covar?
dim(covar)
[1] 140 4
Let’s look at the first few rows.
head(covar)
Sex Genotype Age Batch
123 M C57BL/6J 4 1
181 F C57BL/6J 4 1
191 M C57BL/6J 4 1
193 M C57BL/6J 4 1
221 F C57BL/6J 4 1
225 F C57BL/6J 4 1
Check mouse strains and are there numbers in covar?
table(covar$Genotype)
APOE4 APOE4Trem2 C57BL/6J Trem2
33 35 36 36
Challenge 4
How many samples are in each genotype for each sex and age group?
Solution to Challenge 4
covar %>% group_by(Sex,Genotype,Age) %>% count()
Let’s explore count data.
head(counts,n=5)
gene_ID 116 123 12 181 18421 18422 18424 18425 18427 18428
1 ENSG00000130203 54 37 112 90 16 35 29 36 6 6
2 ENSMUSG00000000001 2347 2648 2302 2585 3291 2256 2791 3029 1880 2298
3 ENSMUSG00000000003 0 0 0 0 0 0 0 0 0 0
4 ENSMUSG00000000028 89 114 60 113 109 72 76 86 73 93
5 ENSMUSG00000000031 47 20 7 18 20 17 12 16 6 12
18429 18430 18431 18432 18465 18469 18472 191 193 19682 19696 19700 20246
1 6 8 21 10 75147 106325 14 47 82 21898 46442 45987 0
2 2677 1884 2023 1745 3554 4040 4438 2494 2996 2545 2736 2851 4255
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 73 88 56 67 123 181 106 107 102 76 96 117 186
5 12 4 19 13 63 18 36 15 67 14 24 41 33
20248 20322 20357 20440 20443 20465 20466 20467 20495 20801 20804 20817
1 0 34116 46491 75376 77544 52 52 30 25511 117185 108849 108754
2 3247 2185 3238 3482 3903 2282 3075 2597 2124 4801 3994 4299
3 0 0 0 0 0 0 0 0 0 0 0 0
4 121 72 84 106 194 111 88 95 60 197 167 163
5 32 22 14 51 30 29 34 11 22 44 32 36
20822 20837 20875 20877 20879 20882 20887 20892 20893 20894 20895 20898 20899
1 70324 78966 78796 74305 80772 94969 4 48 62 32 39 76 12
2 3236 4267 3871 2534 3601 3543 2405 2282 2279 2600 2553 2971 1984
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 201 131 162 118 156 96 64 88 124 122 94 76 57
5 19 15 43 16 13 43 17 17 6 8 13 44 11
22001 22005 22078 22079 22083 22085 22087 22088 22090 22094 22095 22096 22097
1 51479 40252 52870 25029 31474 37812 36563 26525 48147 31663 26470 45566 55277
2 3161 2867 3400 2356 3121 3433 2569 1983 2933 2303 2190 2876 3571
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 89 127 112 89 116 102 127 86 108 84 105 67 124
5 16 10 16 12 24 21 9 9 8 14 20 65 30
22098 22144 22149 22168 22171 22172 22174 221 22514 22523 225 22705 22711
1 34337 36095 42308 34796 41583 30591 47146 41 27832 43621 64 30363 26211
2 2205 2732 2336 2094 2309 2214 2863 2605 2384 2536 3133 2091 1859
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 94 110 70 56 127 104 121 103 89 114 117 108 48
5 10 16 4 9 20 16 39 16 38 36 17 9 12
22712 22723 22746 22754 22755 22770 22869 23110 23156 23161 23168 23170 23184
1 30339 53178 37198 34847 48921 30110 43811 83574 74727 62068 85174 63851 73676
2 2487 2574 2763 2607 2740 3087 3298 4001 3578 3430 3365 2862 3198
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 91 111 95 123 73 105 130 135 134 187 149 126 142
5 24 39 25 3 29 14 45 82 14 12 29 8 33
23409 23410 23412 23414 23415 23450 23451 23454 239 242 251 252 25
1 75553 59847 71130 67167 91016 5 11 14 234 35 43 133 47
2 3537 3043 3514 3189 3944 2472 2226 2884 4137 2394 2945 2800 1989
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 115 134 131 102 194 61 94 137 125 156 80 87 66
5 36 30 34 35 44 20 22 26 7 21 35 100 5
26305 26313 26318 26330 26332 26334 26339 26346 26347 26617 26623 26624 27104
1 0 0 0 1 7 0 0 0 6 36805 38549 42588 39
2 3491 4493 3175 3577 3536 4017 3868 3685 2881 2483 2513 2992 2459
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 132 213 136 149 141 162 121 129 110 69 90 110 88
5 34 69 35 29 21 28 54 43 9 32 13 45 32
27168 27192 27194 27208 27209 272 27354 27355 27356 27357 27385 27386 27392
1 47 33724 40868 55 26 74 85 79 49 61 53 124 43
2 2358 2153 3043 2383 2339 2933 3605 2466 2813 3246 2996 2508 2517
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 87 98 112 107 55 100 124 94 68 150 123 88 97
5 10 12 30 26 20 13 233 29 24 35 20 32 18
27394 27395 27396 27397 27398 27399 27488 27495 27499 27504 27507 27509 27510
1 77 69 37 40 18 52 42890 53940 40501 21691 27115 32449 18986
2 3436 3214 3143 1757 2670 2282 2992 3289 2878 2306 2665 2912 2181
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 146 87 101 52 94 103 92 121 67 79 78 109 56
5 28 13 31 3 10 27 24 42 28 4 14 28 11
27585 27586 27590 27593 27594 27596 27598 27599 27600 27707 27708 27 28129
1 34478 40459 32038 20594 46469 27813 28542 25804 35189 31781 28269 32 104
2 2372 3032 2757 1967 3634 2270 2784 2398 2986 2281 2777 2324 2679
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 59 98 118 97 122 55 93 65 114 106 97 54 138
5 23 48 22 6 39 5 8 5 28 36 32 10 706
288810708 289457705 289461928 289470196 289478142 289482201 289494346
1 35 0 55 23 36 58 0
2 2091 3096 2148 2507 2615 2514 4320
3 0 0 0 0 0 0 0
4 63 116 87 131 55 99 214
5 14 28 16 20 28 8 19
289535121 289576914 289666353 289674340 299 30269 30270 30271 30 344 347
1 64 65 83 10 26 62 26 49 76 41 6
2 2567 3353 2985 2079 2381 3073 2374 2823 4084 3185 3295
3 0 0 0 0 0 0 0 0 0 0 0
4 95 134 109 79 126 102 89 94 128 170 123
5 20 31 30 12 8 29 10 21 49 26 19
35 364 367 393 394 399 3 400 40407 40408 40416 40423 40425 40434
1 35251 20 7 58 19 17 27 10 63779 91374 19 1 0 0
2 2796 3799 3344 5148 3413 2649 2290 3987 3191 4044 3726 3041 2857 3724
3 0 0 0 0 0 0 0 0 0 0 0 0 0 0
4 122 173 186 205 156 103 121 179 100 186 180 161 103 172
5 12 36 32 71 24 17 11 30 17 54 54 45 37 36
40438 40439 40571 40573 40575 40576 40579 40580 40581 40585 40587 40590
1 0 0 128540 78818 55469 59 0 1 0 0 60212 58952
2 3685 2737 6846 4165 3458 2748 4149 3596 4379 3948 3197 3512
3 0 0 0 0 0 0 0 0 0 0 0 0
4 151 104 271 197 139 90 175 156 186 123 101 114
5 53 28 74 67 20 46 54 44 56 44 50 38
40591 40593 40595 40808 40823 40834 40837 40839 40845 40877 40880 42 44
1 78712 0 74833 69807 1 72482 84527 78285 77004 62765 94765 40950 22335
2 4050 3129 3799 3056 4161 3497 3606 3510 4246 3958 4091 2675 2471
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 169 120 139 75 175 148 138 160 146 139 201 131 86
5 68 33 35 19 45 29 39 21 21 62 37 13 34
46611 46618 46621 46623 46630 46642 46647 46648 46649 46650 46651 46652
1 61971 57335 66163 60289 0 68907 107855 0 0 0 0 0
2 3396 2746 2999 3303 3264 3407 4788 3566 3046 3240 4054 3681
3 0 0 0 0 0 0 0 0 0 0 0 0
4 158 149 149 120 111 167 193 140 160 97 135 172
5 20 26 56 29 34 11 41 22 37 6 53 51
46653 46654 46662 46686 46687 46688 46698 46 54 56 57 58 8
1 0 0 59014 0 9 1 58161 53318 41514 42211 37472 45864 77
2 3628 4260 2984 5382 3955 3028 2780 3244 2533 3570 2421 2677 3300
3 0 0 0 0 0 0 0 0 0 0 0 0 0
4 102 173 132 139 233 108 118 162 94 150 71 93 166
5 12 38 11 53 66 33 46 14 11 20 3 19 43
Converting the gene_id as rownames of count matrix
counts <- counts %>% column_to_rownames(.,var="gene_ID") %>% as.data.frame()
How many rows and columns are there in counts?
dim(counts)
[1] 55488 234
In the counts matrix, genes are in rows and samples are in columns.
There are 140 samples in batch 1 as we saw in covar and samples in counts matrix is 234. So, we need to subset counts matrix for samples in covar.
counts <- counts[,colnames(counts) %in% rownames(covar)]
dim(counts)
[1] 55488 140
Now there are count data from 140 samples present in metadata.
Check how many gene_ids are NOT from the mouse genome by searching for the string “MUS” (as in Mus musculus) in the rownames of count matrix
counts[,1:6] %>%
filter(!str_detect(rownames(.), "MUS"))
116 123 12 181 18421 18422
ENSG00000130203 54 37 112 90 16 35
We see entry of gene id “ENSG00000130203”. This is human APOE gene that has been quantified by our MODEL-AD RNA-Seq pipeline.
Here you can also check expression of mouse Apoe.
counts[rownames(counts) %in% "ENSMUSG00000002985",1:10]
116 123 12 181 18421 18422 18424 18425 18427 18428
ENSMUSG00000002985 44173 51114 45209 58427 72939 47959 37884 43401 49936 54232
Let’s visualize the expression of mouse and human APOE gene across all samples using ggplot2 package.
Validation of mouse strains
#First we convert the dataframe to longer format and join our covariates by MouseID
count_tpose <- counts %>%
rownames_to_column(.,var="gene_id") %>%
filter(gene_id %in% c("ENSG00000130203","ENSMUSG00000002985")) %>%
pivot_longer(.,cols = -"gene_id",names_to = "Mouse.ID",values_to="counts") %>% as.data.frame() %>%
left_join(ind_meta %>% mutate("Mouse.ID"=as.character(Mouse.ID)),by="Mouse.ID") %>% as.data.frame()
head(count_tpose)
gene_id Mouse.ID counts Sex Genotype Age Batch
1 ENSG00000130203 116 54 F Trem2 8 1
2 ENSG00000130203 123 37 M C57BL/6J 4 1
3 ENSG00000130203 12 112 M C57BL/6J 8 1
4 ENSG00000130203 181 90 F C57BL/6J 4 1
5 ENSG00000130203 18421 16 F Trem2 12 1
6 ENSG00000130203 18422 35 M Trem2 8 1
#make the age column a factor and re-order the levels
count_tpose$Age <- factor(count_tpose$Age,levels=c(4,8,12))
# rename the gene id to gene symbol
count_tpose$gene_id[count_tpose$gene_id %in% "ENSG00000130203"] <- "Human APOE"
count_tpose$gene_id[count_tpose$gene_id %in% "ENSMUSG00000002985"] <- "Mouse Apoe"
#Create simple box plots showing normalized counts by genotype and time point, faceted by sex.
count_tpose %>%
ggplot(aes(x=Age, y=counts, color=Genotype)) +
geom_boxplot() +
geom_point(position=position_jitterdodge()) +
facet_wrap(~Sex+gene_id) +theme_bw()
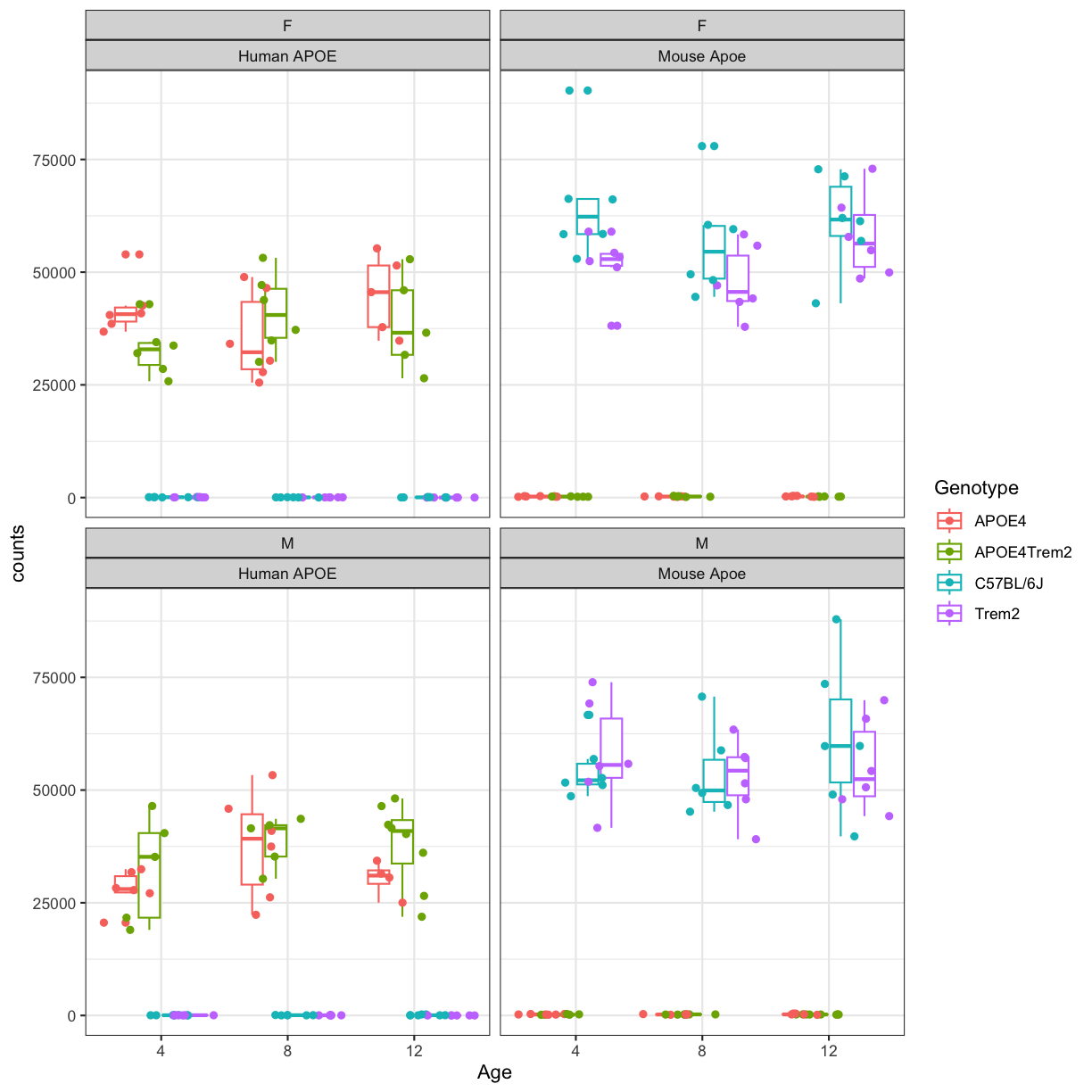
plot of chunk boxplot_APOE4_cohort
You will notice expression of mouse Apoe is higher in samples carrying humanized APOE gene sequences but lower in other samples and vice-versa for human APOE. This also suggest that mouse Apoe gene has been correctly replaced by human APOE gene sequences in APOE4 and APOE4Trem strains. This way we can validated the insertion of human transgene.
As before, we need to merge expression of human APOE and mouse Apoe:
counts[rownames(counts) %in% "ENSMUSG00000002985",] <- counts[rownames(counts) %in% "ENSMUSG00000002985",] + counts[rownames(counts) %in% "ENSG00000130203",]
counts <- counts[!rownames(counts) %in% c("ENSG00000130203"),]
Let’s verify
counts[,1:6] %>%
filter(!str_detect(rownames(.), "MUS"))
[1] 116 123 12 181 18421 18422
<0 rows> (or 0-length row.names)
Formatting the count and metadata for differential analysis
rawdata <- counts[,sort(colnames(counts))]
metadata <- covar[sort(rownames(covar)),]
## renaming column names
colnames(metadata) <- c("sex","genotype","timepoint","Batch")
metadata$sex[metadata$sex %in% "F"] <- "female"
metadata$sex[metadata$sex %in% "M"] <- "male"
Differential Analysis of all groups in LOAD1 cohort
Next, we create a comparison table that has all cases and controls that we would like to compare with each other. Here I have made comparison group for age and sex-matched distinct mouse strains vs C57BL/6J (control mice):
metadata$Group <- paste0(metadata$genotype,"-",metadata$sex,"-",metadata$timepoint)
comparisons <- data.frame(
control=rep(c("C57BL/6J-male-4", "C57BL/6J-female-4", "C57BL/6J-male-8", "C57BL/6J-female-8", "C57BL/6J-male-12", "C57BL/6J-female-12"),3),
case=c("APOE4-male-4", "APOE4-female-4", "APOE4-male-8", "APOE4-female-8", "APOE4-male-12", "APOE4-female-12",
"Trem2-male-4", "Trem2-female-4", "Trem2-male-8", "Trem2-female-8", "Trem2-male-12", "Trem2-female-12",
"APOE4Trem2-male-4", "APOE4Trem2-female-4", "APOE4Trem2-male-8", "APOE4Trem2-female-8", "APOE4Trem2-male-12", "APOE4Trem2-female-12")
)
head(comparisons)
control case
1 C57BL/6J-male-4 APOE4-male-4
2 C57BL/6J-female-4 APOE4-female-4
3 C57BL/6J-male-8 APOE4-male-8
4 C57BL/6J-female-8 APOE4-female-8
5 C57BL/6J-male-12 APOE4-male-12
6 C57BL/6J-female-12 APOE4-female-12
Finally, we implement our DEG function on each comparison and store the result table in a list and data frame:
DE_LOAD1.list <- list()
DE_LOAD1.df <- data.frame()
for (i in 1:nrow(comparisons)){
meta <- metadata[metadata$Group %in% comparisons[i,],]
DEG(rawdata,meta,ref = "C57BL/6J")
#append results in data frame
DE_LOAD1.df <- rbind(DE_LOAD1.df,All_res %>% mutate(model=gsub("-.*$","",comparisons[i,2])[1],sex=unique(meta$sex),age=unique(meta$timepoint)))
#append results in list
DE_LOAD1.list[[i]] <- All_res
names(DE_LOAD1.list)[i] <- paste0(comparisons[i,2])
}
save(DE_LOAD1.df,DE_LOAD1.list,file="../results/DEAnalysis_LOAD1.Rdata")
check number of genes significantly expressed (adjusted p =0.05) in all cases compared to age and sex-matched controls:
degs.up <- map(DE_LOAD1.list, ~length(which(.x$padj<0.05 & .x$log2FoldChange>0)))
degs.down <- map(DE_LOAD1.list, ~length(which(.x$padj<0.05 & .x$log2FoldChange<0)))
deg <- data.frame(comparison=names(degs.up), Up_DEGs.pval.05=as.vector(unlist(degs.up)),Down_DEGs.pval.05=as.vector(unlist(degs.down)))
knitr::kable(deg)
| comparison | Up_DEGs.pval.05 | Down_DEGs.pval.05 |
|---|---|---|
| APOE4-male-4 | 0 | 3 |
| APOE4-female-4 | 0 | 1 |
| APOE4-male-8 | 42 | 103 |
| APOE4-female-8 | 11 | 14 |
| APOE4-male-12 | 0 | 1 |
| APOE4-female-12 | 0 | 3 |
| Trem2-male-4 | 5 | 59 |
| Trem2-female-4 | 5 | 13 |
| Trem2-male-8 | 3 | 29 |
| Trem2-female-8 | 2 | 9 |
| Trem2-male-12 | 118 | 88 |
| Trem2-female-12 | 113 | 172 |
| APOE4Trem2-male-4 | 15 | 8 |
| APOE4Trem2-female-4 | 1 | 2 |
| APOE4Trem2-male-8 | 7 | 32 |
| APOE4Trem2-female-8 | 2 | 1 |
| APOE4Trem2-male-12 | 0 | 2 |
| APOE4Trem2-female-12 | 1 | 4 |
Challenge 5
Save the data for next lesson?
Solution to Challenge 5
save(DE_LOAD1.df,DE_LOAD1.list,file="../result/DEAnalysis_LOAD1.Rdata")
Session Info
sessionInfo()
R version 4.3.0 (2023-04-21)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Monterey 12.6.2
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: America/New_York
tzcode source: internal
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] clusterProfiler_4.8.1 lubridate_1.9.2
[3] forcats_1.0.0 stringr_1.5.0
[5] dplyr_1.1.2 purrr_1.0.1
[7] readr_2.1.4 tidyr_1.3.0
[9] tibble_3.2.1 tidyverse_2.0.0
[11] EnhancedVolcano_1.18.0 ggrepel_0.9.3
[13] GO.db_3.17.0 org.Mm.eg.db_3.17.0
[15] AnnotationDbi_1.62.1 ggplot2_3.4.2
[17] DESeq2_1.40.1 SummarizedExperiment_1.30.2
[19] Biobase_2.60.0 MatrixGenerics_1.12.0
[21] matrixStats_1.0.0 GenomicRanges_1.52.0
[23] GenomeInfoDb_1.36.0 IRanges_2.34.0
[25] S4Vectors_0.38.1 BiocGenerics_0.46.0
[27] knitr_1.43
loaded via a namespace (and not attached):
[1] DBI_1.1.3 bitops_1.0-7 gson_0.1.0
[4] shadowtext_0.1.2 gridExtra_2.3 rlang_1.1.1
[7] magrittr_2.0.3 DOSE_3.26.1 compiler_4.3.0
[10] RSQLite_2.3.1 png_0.1-8 vctrs_0.6.2
[13] reshape2_1.4.4 pkgconfig_2.0.3 crayon_1.5.2
[16] fastmap_1.1.1 XVector_0.40.0 labeling_0.4.2
[19] ggraph_2.1.0 utf8_1.2.3 HDO.db_0.99.1
[22] tzdb_0.4.0 enrichplot_1.20.0 bit_4.0.5
[25] xfun_0.39 zlibbioc_1.46.0 cachem_1.0.8
[28] aplot_0.1.10 jsonlite_1.8.5 blob_1.2.4
[31] highr_0.10 DelayedArray_0.26.3 BiocParallel_1.34.2
[34] tweenr_2.0.2 parallel_4.3.0 R6_2.5.1
[37] RColorBrewer_1.1-3 stringi_1.7.12 GOSemSim_2.26.0
[40] Rcpp_1.0.10 downloader_0.4 Matrix_1.5-4
[43] splines_4.3.0 igraph_1.4.3 timechange_0.2.0
[46] tidyselect_1.2.0 viridis_0.6.3 qvalue_2.32.0
[49] codetools_0.2-19 lattice_0.21-8 plyr_1.8.8
[52] treeio_1.24.1 withr_2.5.0 KEGGREST_1.40.0
[55] evaluate_0.21 gridGraphics_0.5-1 scatterpie_0.2.1
[58] polyclip_1.10-4 Biostrings_2.68.1 ggtree_3.8.0
[61] pillar_1.9.0 ggfun_0.0.9 generics_0.1.3
[64] RCurl_1.98-1.12 hms_1.1.3 tidytree_0.4.2
[67] munsell_0.5.0 scales_1.2.1 glue_1.6.2
[70] lazyeval_0.2.2 tools_4.3.0 data.table_1.14.8
[73] fgsea_1.26.0 locfit_1.5-9.8 graphlayouts_1.0.0
[76] fastmatch_1.1-3 tidygraph_1.2.3 cowplot_1.1.1
[79] grid_4.3.0 ape_5.7-1 colorspace_2.1-0
[82] nlme_3.1-162 patchwork_1.1.2 GenomeInfoDbData_1.2.10
[85] ggforce_0.4.1 cli_3.6.1 fansi_1.0.4
[88] viridisLite_0.4.2 S4Arrays_1.0.4 gtable_0.3.3
[91] yulab.utils_0.0.6 digest_0.6.31 ggplotify_0.1.0
[94] farver_2.1.1 memoise_2.0.1 lifecycle_1.0.3
[97] httr_1.4.6 bit64_4.0.5 MASS_7.3-58.4
Key Points
Validate metadata prior to data analysis.
Use function for repeated differential expression analysis in multiple comparisons.
Mouse-human alignment of transcriptomic signatures
Overview
Teaching: 30 min
Exercises: 10 minQuestions
How well transcriptomic changes we observe in mouse models carrying AD-related mutations align with human AD data?
How do we perfrom corss-species comparison
Objectives
Understand the human AD modules.
Approach to align mouse data to human data
Perform correlation analysis.
visualize the results
Author: Ravi Pandey, Jackson Laboratory
Aligning Human and Mouse Phenotype
ALZHEIMER’S DISEASE (AD) is complex disease, we do not expect these mouse models have complete LOAD (late-onset AD) pathology. We can do MRI as well PET imaging to match back to human imaging study ADNI, we can do neuropathology, finally we can do lots of Genomics, proteomics, and metabolomics. These omics study allows us to do real direct homology comparison between human and mouse as genes are overwhemly shared between these two species.
Overview of Human transcriptomic data
Three independent human brain transcriptome studies ROSMAP [Religious Orders Study and the Memory and Aging Project], MSSM [Mount Sinai School of Medicine], and Mayo collected human postmortem brain RNA-seq data from seven distinct regions: dorsolateral prefrontal cortex (DLPFC), temporal cortex (TCX), inferior frontal gyrus (IFG), superior temporal gyrus (STG), frontal pole (FP), parahippocampal gyrus (PHG), and cerebellum (CBE),
These postmortem samples are generally balanced for AD, MCI, and non-effected controls. This really give us broad assessment how AD as affecetd multiple brain region in 3 different population around the US.
Overview of Human Consensus RNA-Seq Coexpression Modules
The Accelerating Medicines Partnership-Alzheimer’s Disease (AMP-AD) Consortium has generated RNA-seq profiles from more than 1,200 human brains and is applying systems biology approaches toward the goal of elucidating AD mechanisms and potential therapeutic targets.
Wan, et al. performed meta analysis including all available AMP-AD RNA-seq datasets and systematically define correspondences between gene expression changes associated with AD in human brains. Briefly, Wan, et al. performed library normalization and covariate adjustments for each human study separately using fixed/mixed effects modeling to account for batch effects. Among the 2978 AMP-AD modules identified across all tissues, 660 modules were selected which showed an enrichment for at least one AD-specific differential expressed gene set from the meta-analysis in cases compared to controls.
Next, they performed multi method co-expression network analysis followed by differential analysis and found 30 co-expression modules related LOAD pathology from human cohort study. Among the 30 aggregate co-expression modules, five consensus clusters have been described by Wan, et al. These consensus clusters consist of a subset of modules which are associated with similar AD related changes across the multiple studies and brain regions. Further, they looked for enrichment of cell type signature in these modules using expression-weighted cell type enrichment analysis Skene and Grant, 2016 as well as applied functional annotation to these modules.
First module block enriched in astrocytes, next block is enriched in endothelial and microglial genes suggesting strong inflammation component, next block in strongly enriched in neuron suggesting neurodegeneration, next is enriched in oligodendrocytes and glial genes suggesting myelination and finally mixed modules that have things to do like stress response and response to unfolded proteins. Stress response not cell specific,so they may be throughout many cells in brain.
Here we are showing matrix view of gene content overlap between these module, and you can see few strongly overlapping group of modules, implicating similar pathology in different studies in different brain regions.
Reading AMP-AD modules data
You can download data on the 30 human AMP-AD co-expression modules was obtained from the Synapse data repository (https://www.synapse.org/#!Synapse:syn11932957/tables/; SynapseID: syn11932957).
query <- synTableQuery("SELECT * FROM syn11932957")
module_table <- read.table(query$filepath, sep = ",",header = TRUE)
Let’s look at module table
head(module_table)
ROW_ID ROW_VERSION GeneID Module method
1 0 0 ENSG00000168439 DLPFCturquoise aggregate
2 1 0 ENSG00000086061 DLPFCturquoise aggregate
3 2 0 ENSG00000204389 DLPFCturquoise aggregate
4 3 0 ENSG00000114416 DLPFCturquoise aggregate
5 4 0 ENSG00000110172 DLPFCturquoise aggregate
6 5 0 ENSG00000099622 DLPFCturquoise aggregate
ModuleName brainRegion ModuleNameFull
1 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
2 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
3 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
4 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
5 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
6 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
external_gene_name
1 STIP1
2 DNAJA1
3 HSPA1A
4 FXR1
5 CHORDC1
6 CIRBP
Here you see total 9 columns in this table. Column of our interest are: *Colum 2: human ensembl gene ID, *column 3: module name in which gene is clustered and *column 7: is brain tissue. *column 9: is gene names.
How many distinct modules are in table?
length(unique(module_table$Module))
[1] 30
What are the name of modules?
unique(module_table$Module)
[1] "DLPFCturquoise" "DLPFCblue" "DLPFCbrown" "DLPFCyellow"
[5] "CBEturquoise" "CBEblue" "CBEbrown" "CBEyellow"
[9] "TCXturquoise" "TCXblue" "TCXbrown" "TCXyellow"
[13] "TCXgreen" "IFGturquoise" "IFGblue" "IFGbrown"
[17] "IFGyellow" "STGturquoise" "STGblue" "STGbrown"
[21] "STGyellow" "PHGturquoise" "PHGblue" "PHGbrown"
[25] "PHGyellow" "PHGgreen" "FPturquoise" "FPblue"
[29] "FPbrown" "FPyellow"
and how many genes are in each module?
table(module_table$Module)
CBEblue CBEbrown CBEturquoise CBEyellow DLPFCblue
4509 504 1977 1739 1751
DLPFCbrown DLPFCturquoise DLPFCyellow FPblue FPbrown
882 2489 3019 1991 1289
FPturquoise FPyellow IFGblue IFGbrown IFGturquoise
1001 4426 2885 4673 1456
IFGyellow PHGblue PHGbrown PHGgreen PHGturquoise
743 3733 2123 1151 1195
PHGyellow STGblue STGbrown STGturquoise STGyellow
910 1171 3414 2404 1799
TCXblue TCXbrown TCXgreen TCXturquoise TCXyellow
1713 1851 2766 1131 2013
You can also visualize this as bar plot using ggplot2 package.
ggplot(module_table,aes(x=Module)) +
geom_bar() +
theme_bw() +
theme(axis.text.x = ggplot2::element_text(angle = 90, hjust = 0, size = 12))
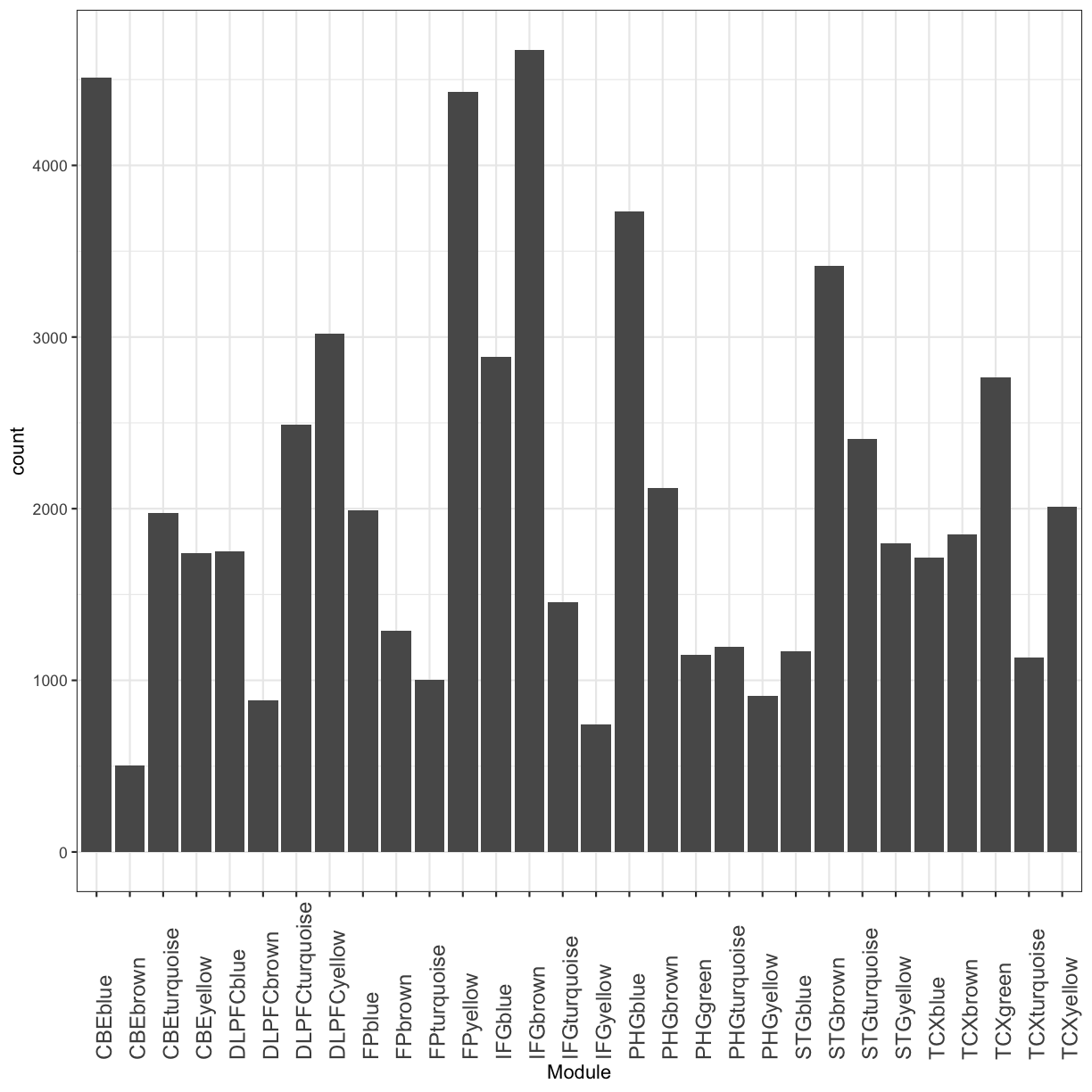
plot of chunk module_nGenes
Challenge 1
What are other ways to count genes in each module?
Solution to Challenge 1
dplyr::count(module_table ,Module)
We can also check total unique genes in table
length(unique((module_table$GeneID)))
[1] 17033
You can also check which modules come from which brain region.
unique(module_table[c("Module", "brainRegion")])
Module brainRegion
1 DLPFCturquoise DLPFC
2490 DLPFCblue DLPFC
4241 DLPFCbrown DLPFC
5123 DLPFCyellow DLPFC
8142 CBEturquoise CBE
10119 CBEblue CBE
14628 CBEbrown CBE
15132 CBEyellow CBE
16871 TCXturquoise TCX
18002 TCXblue TCX
19715 TCXbrown TCX
21566 TCXyellow TCX
23579 TCXgreen TCX
26345 IFGturquoise IFG
27801 IFGblue IFG
30686 IFGbrown IFG
35359 IFGyellow IFG
36102 STGturquoise STG
38506 STGblue STG
39677 STGbrown STG
43091 STGyellow STG
44890 PHGturquoise PHG
46085 PHGblue PHG
49818 PHGbrown PHG
51941 PHGyellow PHG
52851 PHGgreen PHG
54002 FPturquoise FP
55003 FPblue FP
56994 FPbrown FP
58283 FPyellow FP
Mouse-Human orthologous gene conversion
In module table, we have human ENSEMBL ids and gene names. But we will need corresponding mouse gene name, so that we can compare with our results from mouse models. To do this, we are going to add mouse orthologous gene names corresponding to human ENSEMBL id. Mouse orthologs for human genes were extracted using the HCOP tool (The HGNC Comparison of Orthology Predictions) by Wan, et al.. We are going to read that table from the Synapse data repository (https://doi.org/10.7303/syn17010253.1,synapse id:syn17010253)
mouse.human.ortho <- fread(synapser::synGet("syn17010253")$path,check.names = F,header=T)
Let’s see top rows of this ortholog table:
head(mouse.human.ortho)
human_entrez_gene human_ensembl_gene hgnc_id
1: - ENSG00000274059 -
2: - ENSG00000212595 -
3: - ENSG00000277418 -
4: - ENSG00000274759 -
5: - ENSG00000274663 -
6: - ENSG00000277488 -
human_name human_symbol human_chr
1: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA 1
2: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA X
3: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA 8
4: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA 4
5: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA 17
6: 5S ribosomal RNA [Source:RFAM;Acc:RF00001] 5S_rRNA 17
human_assert_ids mouse_entrez_gene mouse_ensembl_gene mgi_id
1: ENSG00000274059 - ENSMUSG00000089601 MGI:5451871
2: ENSG00000212595 - ENSMUSG00000088132 MGI:5452363
3: ENSG00000277418 - ENSMUSG00000088814 MGI:5452162
4: ENSG00000274759 - ENSMUSG00000084431 MGI:4421893
5: ENSG00000274663 - ENSMUSG00000084588 MGI:4421913
6: ENSG00000277488 - ENSMUSG00000084588 MGI:4421913
mouse_name mouse_symbol mouse_chr mouse_assert_ids support
1: predicted gene, 22094 Gm22094 - ENSMUSG00000089601 Ensembl
2: predicted gene, 22586 Gm22586 - ENSMUSG00000088132 Ensembl
3: predicted gene, 22385 Gm22385 - ENSMUSG00000088814 Ensembl
4: nuclear encoded rRNA 5S 48 n-R5s48 - ENSMUSG00000084431 Ensembl
5: nuclear encoded rRNA 5S 68 n-R5s68 - ENSMUSG00000084588 Ensembl
6: nuclear encoded rRNA 5S 68 n-R5s68 - ENSMUSG00000084588 Ensembl
Add mouse gene names from ortholog table to module table by matching human ENSEMBL ids from both tables .
module_table$Mouse_gene_name <- mouse.human.ortho$mouse_symbol[match(module_table$GeneID,mouse.human.ortho$human_ensembl_gene)]
head(module_table)
ROW_ID ROW_VERSION GeneID Module method
1 0 0 ENSG00000168439 DLPFCturquoise aggregate
2 1 0 ENSG00000086061 DLPFCturquoise aggregate
3 2 0 ENSG00000204389 DLPFCturquoise aggregate
4 3 0 ENSG00000114416 DLPFCturquoise aggregate
5 4 0 ENSG00000110172 DLPFCturquoise aggregate
6 5 0 ENSG00000099622 DLPFCturquoise aggregate
ModuleName brainRegion ModuleNameFull
1 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
2 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
3 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
4 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
5 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
6 aggregateDLPFCturquoise DLPFC aggregateDLPFCturquoiseDLPFC
external_gene_name Mouse_gene_name
1 STIP1 Stip1
2 DNAJA1 Dnaja1
3 HSPA1A Hspa1a
4 FXR1 Fxr1
5 CHORDC1 Chordc1
6 CIRBP Cirbp
We will only keep column of our interest and non-empty entries:
ampad_modules <- module_table %>%
distinct(tissue = brainRegion, module = Module, gene = GeneID, Mouse_gene_name) %>%
filter(Mouse_gene_name != "")
head(ampad_modules)
tissue module gene Mouse_gene_name
1 DLPFC DLPFCturquoise ENSG00000168439 Stip1
2 DLPFC DLPFCturquoise ENSG00000086061 Dnaja1
3 DLPFC DLPFCturquoise ENSG00000204389 Hspa1a
4 DLPFC DLPFCturquoise ENSG00000114416 Fxr1
5 DLPFC DLPFCturquoise ENSG00000110172 Chordc1
6 DLPFC DLPFCturquoise ENSG00000099622 Cirbp
Reading differential expression result of human data from meta-analysis
Differential expression meta-analysis of reprocessed RNASeq data from AMP-AD (all 7 brain regions). LogFC values for human transcripts were obtained via the AMP-AD knowledge portal(https://www.synapse.org/#!Synapse:syn11180450; SynapseID: syn11180450).
ampad_modules_raw <- fread(synapser::synGet("syn11180450")$path,check.names = F,header=T)
Let’s check the data
head(ampad_modules_raw)
Model Tissue Comparison ensembl_gene_id logFC CI.L
1: SourceDiagnosis CBE AD-CONTROL ENSG00000078043 -0.4455482 -0.5474320
2: SourceDiagnosis CBE AD-CONTROL ENSG00000205302 0.4534512 0.3452585
3: SourceDiagnosis CBE AD-CONTROL ENSG00000134982 0.5778206 0.4383669
4: SourceDiagnosis CBE AD-CONTROL ENSG00000173230 0.5924321 0.4493164
5: SourceDiagnosis CBE AD-CONTROL ENSG00000115204 -0.3426223 -0.4257147
6: SourceDiagnosis CBE AD-CONTROL ENSG00000163867 -0.3176858 -0.3965217
CI.R AveExpr t P.Value adj.P.Val B Direction
1: -0.3436645 1.3320969 -8.592843 1.206969e-16 2.053175e-12 27.12967 DOWN
2: 0.5616439 2.9361993 8.235175 1.699316e-15 1.445353e-11 24.54943 UP
3: 0.7172742 3.5498628 8.141480 3.365041e-15 1.530863e-11 23.85060 UP
4: 0.7355477 3.0759381 8.133894 3.599700e-15 1.530863e-11 23.81776 UP
5: -0.2595299 2.2634337 -8.102128 4.512223e-15 1.535148e-11 23.62730 DOWN
6: -0.2388499 0.4404036 -7.918097 1.695179e-14 4.806115e-11 22.34035 DOWN
hgnc_symbol percentage_gc_content gene.length Sex Study
1: PIAS2 38.59 17527 ALL MAYO
2: SNX2 36.08 4254 ALL MAYO
3: APC 37.63 12440 ALL MAYO
4: GOLGB1 38.07 11865 ALL MAYO
5: MPV17 50.33 5625 ALL MAYO
6: ZMYM6 38.80 9591 ALL MAYO
Data came from how many tissues?
unique(ampad_modules_raw$Tissue)
[1] "CBE" "TCX" "FP" "IFG" "PHG" "STG" "DLPFC"
We see that data is from all 7 brain regions.
AMP-AD data has been processed many ways and using different models and comparisons. Let’s see how many ways data has been analyzed,
unique(ampad_modules_raw$Comparison)
[1] "AD-CONTROL" "PATH_AGE-CONTROL"
[3] "PSP-CONTROL" "AD-PATH_AGE"
[5] "AD-OTHER" "OTHER-CONTROL"
[7] "2-0" "2-1"
[9] "1-0" "CDR"
[11] "4-1" "4-2"
[13] "FEMALE-MALE" "AD-CONTROL.IN.FEMALE-MALE"
[15] "AOD"
For our analysis, we need data for “Diagnosis” model and comparison between cases vs controls. So, we subset the logFC data for these conditions. Also, we need only three columns: “Tissue”,”Gene” and “logFC”. So, we will filter and subset the data.
ampad_fc <- ampad_modules_raw %>%
as_tibble() %>%
filter(Model == "Diagnosis", Comparison == "AD-CONTROL") %>%
dplyr::select(tissue = Tissue, gene = ensembl_gene_id, ampad_fc = logFC)
Combine with modules so correlation can be done per module
Next, we will combine fold change table ampad_fc and module table ampad_modules. First, look at both tables to check how can we merge them together?
head(ampad_fc)
# A tibble: 6 × 3
tissue gene ampad_fc
<chr> <chr> <dbl>
1 CBE ENSG00000085224 0.363
2 CBE ENSG00000131016 0.481
3 CBE ENSG00000078043 -0.361
4 CBE ENSG00000243943 -0.257
5 CBE ENSG00000093167 -0.477
6 CBE ENSG00000058272 0.330
head(ampad_modules)
tissue module gene Mouse_gene_name
1 DLPFC DLPFCturquoise ENSG00000168439 Stip1
2 DLPFC DLPFCturquoise ENSG00000086061 Dnaja1
3 DLPFC DLPFCturquoise ENSG00000204389 Hspa1a
4 DLPFC DLPFCturquoise ENSG00000114416 Fxr1
5 DLPFC DLPFCturquoise ENSG00000110172 Chordc1
6 DLPFC DLPFCturquoise ENSG00000099622 Cirbp
In both tables, common columns are “gene” and “tissue”. So we will merge both datasets using these two columns. Reminder: Every gene can be present in multiple brain regions but only one module form any brain region. Let’s check that:
ampad_modules[ampad_modules$gene %in% "ENSG00000168439",]
tissue module gene Mouse_gene_name
1 DLPFC DLPFCturquoise ENSG00000168439 Stip1
9376 CBE CBEblue ENSG00000168439 Stip1
17627 TCX TCXbrown ENSG00000168439 Stip1
31448 STG STGturquoise ENSG00000168439 Stip1
40130 PHG PHGblue ENSG00000168439 Stip1
48317 FP FPblue ENSG00000168439 Stip1
We can see that this gene is present in six distinct modules and all modules are from different brain regions. You can do for any other gene as well.
Let’s merge using inner_join function from tidyverse:
ampad_modules_fc <- ampad_modules %>%
inner_join(ampad_fc, by = c("gene", "tissue")) %>%
dplyr::select(symbol = Mouse_gene_name, module, ampad_fc)
head(ampad_modules_fc)
symbol module ampad_fc
1 Stip1 DLPFCturquoise 0.03342379
2 Dnaja1 DLPFCturquoise 0.02350154
3 Hspa1a DLPFCturquoise 0.10220020
4 Fxr1 DLPFCturquoise 0.03842087
5 Chordc1 DLPFCturquoise 0.06350503
6 Cirbp DLPFCturquoise -0.24214640
We will use ampad_modules_fc dataset to compare with log fold change data from mouse models.
Preparing module information for correlation plot
mod <- c("TCXblue","PHGyellow","IFGyellow","DLPFCblue","CBEturquoise","STGblue","PHGturquoise","IFGturquoise","TCXturquoise","FPturquoise","IFGbrown","STGbrown","DLPFCyellow","TCXgreen","FPyellow","CBEyellow","PHGbrown","DLPFCbrown","STGyellow","PHGgreen","CBEbrown","TCXyellow","IFGblue","FPblue","FPbrown","CBEblue","DLPFCturquoise","TCXbrown","STGturquoise","PHGblue")
cluster_a <- tibble(
module = c("TCXblue", "PHGyellow", "IFGyellow"),
cluster = "Consensus Cluster A (ECM organization)",
cluster_label = "Consensus Cluster A\n(ECM organization)"
)
cluster_b <- tibble(
module = c("DLPFCblue", "CBEturquoise", "STGblue", "PHGturquoise", "IFGturquoise", "TCXturquoise", "FPturquoise"),
cluster = "Consensus Cluster B (Immune system)",
cluster_label = "Consensus Cluster B\n(Immune system)"
)
cluster_c <- tibble(
module = c("IFGbrown", "STGbrown", "DLPFCyellow", "TCXgreen", "FPyellow", "CBEyellow", "PHGbrown"),
cluster = "Consensus Cluster C (Neuronal system)",
cluster_label = "Consensus Cluster C\n(Neuronal system)"
)
cluster_d <- tibble(
module = c("DLPFCbrown", "STGyellow", "PHGgreen", "CBEbrown", "TCXyellow", "IFGblue", "FPblue"),
cluster = "Consensus Cluster D (Cell Cycle, NMD)",
cluster_label = "Consensus Cluster D\n(Cell Cycle, NMD)"
)
cluster_e <- tibble(
module = c("FPbrown", "CBEblue", "DLPFCturquoise", "TCXbrown", "STGturquoise", "PHGblue"),
cluster = "Consensus Cluster E (Organelle Biogensis, Cellular stress response)",
cluster_label = "Consensus Cluster E\n(Organelle Biogenesis,\nCellular stress response)"
)
module_clusters <- cluster_a %>%
bind_rows(cluster_b) %>%
bind_rows(cluster_c) %>%
bind_rows(cluster_d) %>%
bind_rows(cluster_e) %>%
mutate(cluster_label = fct_inorder(cluster_label))
head(module_clusters)
# A tibble: 6 × 3
module cluster cluster_label
<chr> <chr> <fct>
1 TCXblue Consensus Cluster A (ECM organization) "Consensus Cluster A\n(EC…
2 PHGyellow Consensus Cluster A (ECM organization) "Consensus Cluster A\n(EC…
3 IFGyellow Consensus Cluster A (ECM organization) "Consensus Cluster A\n(EC…
4 DLPFCblue Consensus Cluster B (Immune system) "Consensus Cluster B\n(Im…
5 CBEturquoise Consensus Cluster B (Immune system) "Consensus Cluster B\n(Im…
6 STGblue Consensus Cluster B (Immune system) "Consensus Cluster B\n(Im…
save(ampad_modules_fc,module_clusters,mod,file="../data/AMPADModuleData_Correlation.RData")
Correlation between mouse models and human AD modules
There are two approaches that we adopted to compute correlation between mouse data with human AD modules:
- Compare change in expression in Human AD cases with change in expression in mouse models for each orthologous gene in a given module
- LogFC(h) = log fold change in transcript expression of human AD patients compared to control patients.
- LogFC(m) = log fold change in transcript expression of mouse AD models compare to control mouse models. \(cor.test(LogFC(h), LogFC(m))\)
- Compare Human AD to mouse genetic effects for each orthologous gene in a given module
- h = human gene expression (Log2 RNA-seq Fold Change control/AD)
- β = mouse gene expression effect from linear regression model (Log2 RNA-seq TPM) \(cor.test(LogFC(h), β)\)
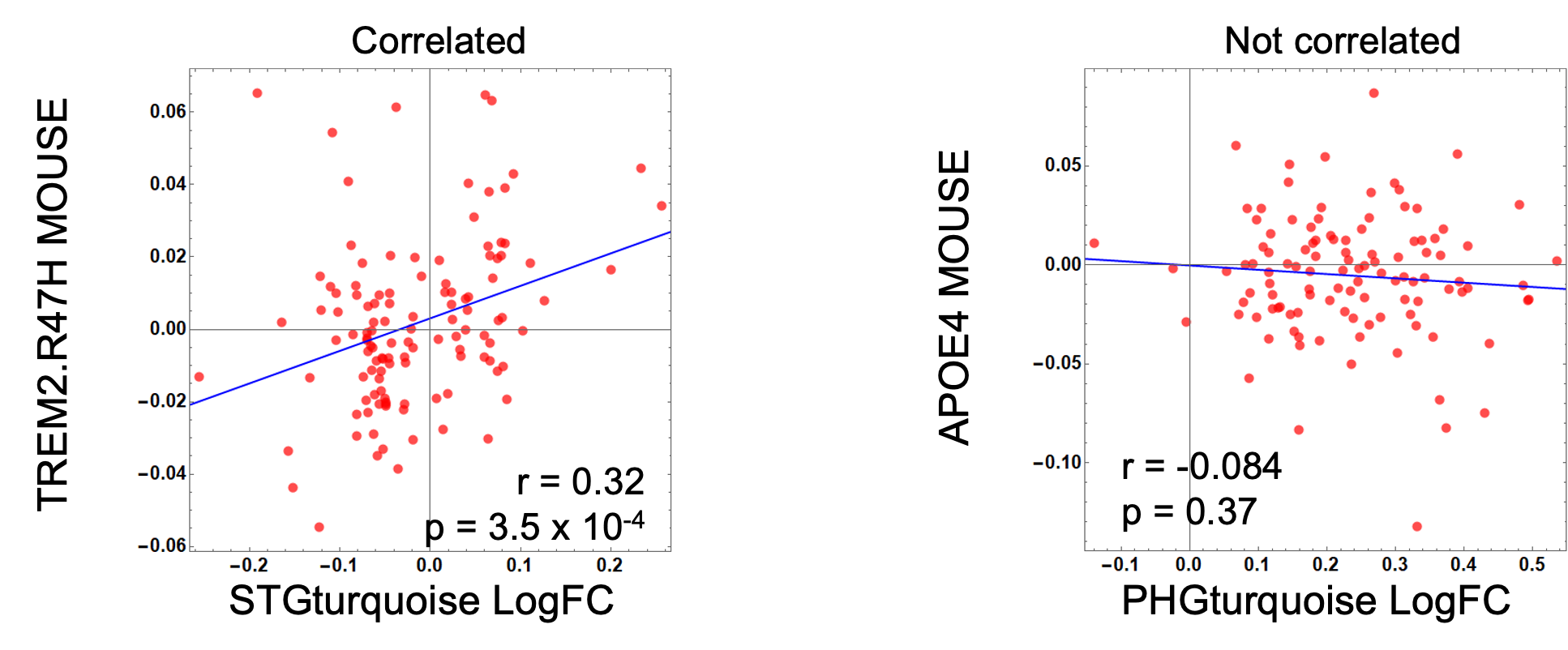
These appraoches allow us to assess directional coherence between AMP-AD modules and the effects of genetic perturbations in mice. In this lesson, we are going to use first approach. Let’s start!
Step0: Reading Gene Expression Count matrix from Previous Lesson
We first read the result table saved after differential analysis in last lesson. We start with 5XFAD mouse model to understand all required steps to perform correlation with human AD modules.
load("../results/DEAnalysis_5XFAD.Rdata")
We can also load AMP-AD module data.
load("../data/AMPADModuleData_Correlation.RData")
Step1: Measure the correlation between mouse models for each sex at each age and AMP-AD modules using common genes from both datasets
We compute pearson correlation between changes in expression for each gene in a given module (log fold change for cases minus controls) with each mouse model (log fold change of the 5XFAD mice minus sex and age-matched B6 mice).
First, we add both mouse DE_5xFAD.df and human ampad_modules_fc log fold change datasets for all genes.
model_vs_ampad <- DE_5xFAD.df %>%
inner_join(ampad_modules_fc, by = c("symbol"),multiple = "all")
head(model_vs_ampad)
symbol EntrezGene baseMean log2FoldChange lfcSE stat pvalue
1 Gnai3 14679 3707.5316 -0.02308587 0.0381646 -0.6049026 0.5452437
2 Gnai3 14679 3707.5316 -0.02308587 0.0381646 -0.6049026 0.5452437
3 Gnai3 14679 3707.5316 -0.02308587 0.0381646 -0.6049026 0.5452437
4 Gnai3 14679 3707.5316 -0.02308587 0.0381646 -0.6049026 0.5452437
5 Scml2 107815 126.8241 0.08939456 0.1377406 0.6490066 0.5163341
6 Scml2 107815 126.8241 0.08939456 0.1377406 0.6490066 0.5163341
padj model sex age module ampad_fc
1 0.9999518 5xFAD male 4 mo TCXblue 0.18017910
2 0.9999518 5xFAD male 4 mo IFGturquoise -0.01064322
3 0.9999518 5xFAD male 4 mo STGblue -0.01063594
4 0.9999518 5xFAD male 4 mo FPturquoise -0.03142185
5 0.9999518 5xFAD male 4 mo STGturquoise 0.07891395
6 0.9999518 5xFAD male 4 mo PHGblue 0.09451007
Next, we create a list-columns of data frame using nest function of tidyverse package. Nesting is implicitly a summarising operation: you get one row for each group defined by the non-nested columns.
df <- model_vs_ampad %>%
dplyr::select(module, model, sex, age, symbol, log2FoldChange, ampad_fc) %>%
group_by(module, model, sex,age) %>%
nest(data = c(symbol, log2FoldChange, ampad_fc))
head(df)
# A tibble: 6 × 5
# Groups: module, model, sex, age [6]
module model sex age data
<chr> <chr> <chr> <chr> <list>
1 TCXblue 5xFAD male 4 mo <tibble [1,462 × 3]>
2 IFGturquoise 5xFAD male 4 mo <tibble [1,310 × 3]>
3 STGblue 5xFAD male 4 mo <tibble [1,092 × 3]>
4 FPturquoise 5xFAD male 4 mo <tibble [927 × 3]>
5 STGturquoise 5xFAD male 4 mo <tibble [1,748 × 3]>
6 PHGblue 5xFAD male 4 mo <tibble [2,841 × 3]>
total number of groups in data table
dim(df)
[1] 180 5
Let’s check first row:
head(df[1,]$data)
[[1]]
# A tibble: 1,462 × 3
symbol log2FoldChange ampad_fc
<chr> <dbl> <dbl>
1 Gnai3 -0.0231 0.180
2 Gna12 -0.00445 0.444
3 Sdhd -0.00152 0.0631
4 Lhx2 -0.0959 0.252
5 Gmpr 0.0105 0.801
6 Tpd52l1 -0.103 0.714
7 Cdh4 -0.0111 0.109
8 Dbt -0.0460 -0.0103
9 Tbrg4 0.00746 -0.129
10 Galnt1 -0.0105 0.191
# ℹ 1,452 more rows
Next, we compute correlation coefficients using cor.test function built in R as following:
cor.df <- df %>%
mutate(
cor_test = map(data, ~ cor.test(.x[["log2FoldChange"]], .x[["ampad_fc"]], method = "pearson")),
estimate = map_dbl(cor_test, "estimate"),
p_value = map_dbl(cor_test, "p.value")
) %>%
ungroup() %>%
dplyr::select(-cor_test)
head(cor.df)
# A tibble: 6 × 7
module model sex age data estimate p_value
<chr> <chr> <chr> <chr> <list> <dbl> <dbl>
1 TCXblue 5xFAD male 4 mo <tibble [1,462 × 3]> 0.0681 9.19e- 3
2 IFGturquoise 5xFAD male 4 mo <tibble [1,310 × 3]> 0.227 9.10e-17
3 STGblue 5xFAD male 4 mo <tibble [1,092 × 3]> 0.284 9.55e-22
4 FPturquoise 5xFAD male 4 mo <tibble [927 × 3]> 0.103 1.72e- 3
5 STGturquoise 5xFAD male 4 mo <tibble [1,748 × 3]> -0.0538 2.44e- 2
6 PHGblue 5xFAD male 4 mo <tibble [2,841 × 3]> -0.00934 6.19e- 1
Step2: add signifcant correlations and join module cluster information to correlation table
model_module <- cor.df %>%
mutate(significant = p_value < 0.05) %>%
left_join(module_clusters, by = "module") %>%
dplyr::select(cluster, cluster_label, module, model, sex,age,correlation = estimate, p_value, significant)
head(model_module)
# A tibble: 6 × 9
cluster cluster_label module model sex age correlation p_value
<chr> <fct> <chr> <chr> <chr> <chr> <dbl> <dbl>
1 Consensus Cluster… "Consensus C… TCXbl… 5xFAD male 4 mo 0.0681 9.19e- 3
2 Consensus Cluster… "Consensus C… IFGtu… 5xFAD male 4 mo 0.227 9.10e-17
3 Consensus Cluster… "Consensus C… STGbl… 5xFAD male 4 mo 0.284 9.55e-22
4 Consensus Cluster… "Consensus C… FPtur… 5xFAD male 4 mo 0.103 1.72e- 3
5 Consensus Cluster… "Consensus C… STGtu… 5xFAD male 4 mo -0.0538 2.44e- 2
6 Consensus Cluster… "Consensus C… PHGbl… 5xFAD male 4 mo -0.00934 6.19e- 1
# ℹ 1 more variable: significant <lgl>
Step3: Create a dataframe to use as input for plotting the results
order.model <- c("5xFAD (Male)", "5xFAD (Female)")
correlation_for_plot <- model_module %>%
arrange(cluster) %>%
mutate(
module = factor(module,levels=mod),
model_sex = glue::glue("{model} ({str_to_title(sex)})"),
) %>%
arrange(model_sex) %>%
mutate(
model_sex = factor(model_sex,levels=order.model),
model_sex = fct_rev(model_sex),
)
head(correlation_for_plot)
# A tibble: 6 × 10
cluster cluster_label module model sex age correlation p_value significant
<chr> <fct> <fct> <chr> <chr> <chr> <dbl> <dbl> <lgl>
1 Consen… "Consensus C… TCXbl… 5xFAD fema… 4 mo 0.114 1.31e-5 TRUE
2 Consen… "Consensus C… PHGye… 5xFAD fema… 4 mo 0.201 9.14e-9 TRUE
3 Consen… "Consensus C… IFGye… 5xFAD fema… 4 mo 0.0757 5.12e-2 FALSE
4 Consen… "Consensus C… TCXbl… 5xFAD fema… 6 mo 0.115 9.69e-6 TRUE
5 Consen… "Consensus C… PHGye… 5xFAD fema… 6 mo 0.0668 5.87e-2 FALSE
6 Consen… "Consensus C… IFGye… 5xFAD fema… 6 mo 0.0707 6.86e-2 FALSE
# ℹ 1 more variable: model_sex <fct>
Visualizing the Correlation plot
Now, we will use above matrix and visualize the correlation results using ggplot2 package.
data <- correlation_for_plot
range(correlation_for_plot$correlation)
[1] -0.1749441 0.3684976
ggplot2::ggplot() +
ggplot2::geom_tile(data = data, ggplot2::aes(x = .data$module, y = .data$model_sex), colour = "black", fill = "white") +
ggplot2::geom_point(data = dplyr::filter(data), ggplot2::aes(x = .data$module, y = .data$model_sex, colour = .data$correlation, size = abs(.data$correlation))) +
ggplot2::geom_point(data = dplyr::filter(data, .data$significant), ggplot2::aes(x = .data$module, y = .data$model_sex, colour = .data$correlation),color="black",shape=0,size=9) +
ggplot2::scale_x_discrete(position = "top") +
ggplot2::scale_size(guide = "none", limits = c(0, 0.4)) +
ggplot2::scale_color_gradient2(limits = c(-0.4, 0.4), breaks = c(-0.4, 0, 0.4), low = "#85070C", high = "#164B6E", name = "Correlation", guide = ggplot2::guide_colorbar(ticks = FALSE)) +
ggplot2::labs(x = NULL, y = NULL) +
ggplot2::facet_grid( rows = dplyr::vars(.data$age),cols = dplyr::vars(.data$cluster_label), scales = "free", space = "free",switch = 'y') +
ggplot2::theme(
strip.text.x = ggplot2::element_text(size = 10,colour = c("black")),
strip.text.y.left = ggplot2::element_text(angle = 0,size = 12),
axis.ticks = ggplot2::element_blank(),
axis.text.x = ggplot2::element_text(angle = 90, hjust = 0, size = 12),
axis.text.y = ggplot2::element_text(angle = 0, size = 12),
panel.background = ggplot2::element_blank(),
plot.title = ggplot2::element_text(angle = 0, vjust = -54, hjust = 0.03,size=12,face="bold"),
plot.title.position = "plot",
panel.grid = ggplot2::element_blank(),
legend.position = "right"
)
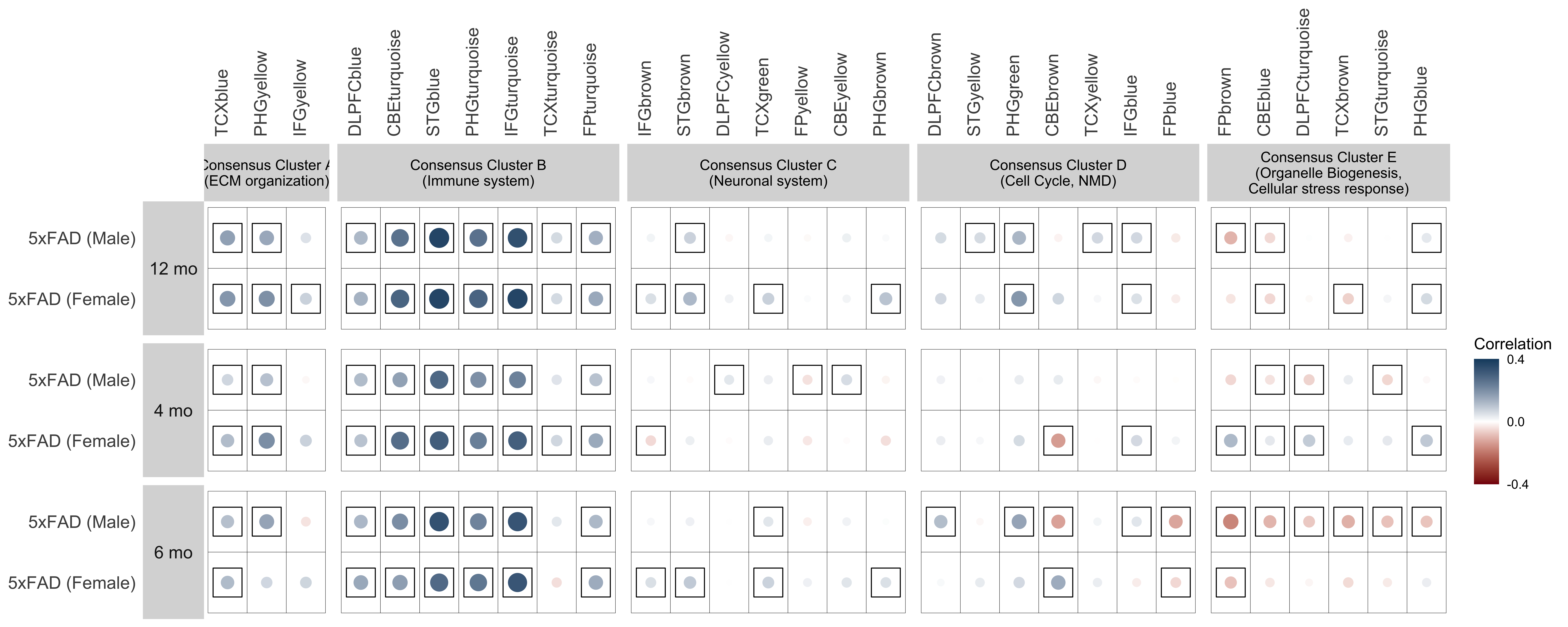
In above plot, top row represent 30 AMP-AD modules grouped into 5 consensus clusters describing the major functional groups of AD-related alterations and left column represent mouse models. Positive correlations are shown in blue and negative correlations in red. Color intensity and size of the circles are proportional to the correlation coefficient. Black square around dots represent significant correlation at p-value=0.05 and non-significant correlations are left blank.
Male and female 5XFAD mice display gene expression alterations across all five consensus clusters, with the most pronounced alterations observed in Consensus Cluster B, which consists of immune system pathways.
We also saw in Volcano plot that immune-related genes were signifcantly overexpressed in the 5XFAD model. This significant positive correlation suggest that 5XFAD model capture inflammatory changes observed in human AD patients.
Combing all steps into a function to perform correlation analysis
We can combine all above steps to perform correlation analysis into one function to use for other data by just inputting log fod change matrix obtained from differential analysis.
corr_function <- function(mydat)
{
model_vs_ampad <- mydat %>%
inner_join(ampad_modules_fc, by = c("symbol"),multiple = "all") %>%
dplyr::select(module, model, sex, age, symbol, log2FoldChange, ampad_fc) %>%
group_by(module, model, sex,age) %>%
nest(data = c(symbol, log2FoldChange, ampad_fc)) %>%
mutate(
cor_test = map(data, ~ cor.test(.x[["log2FoldChange"]], .x[["ampad_fc"]], method = "pearson")),
estimate = map_dbl(cor_test, "estimate"),
p_value = map_dbl(cor_test, "p.value")
) %>%
ungroup() %>%
dplyr::select(-cor_test)
model_module <- model_vs_ampad %>%
mutate(significant = p_value < 0.05) %>%
left_join(module_clusters, by = "module") %>%
dplyr::select(cluster, cluster_label, module, model, sex,age,correlation = estimate, p_value, significant)
correlation_for_plot <- model_module %>%
arrange(cluster) %>%
mutate(
module = factor(module,levels=mod),
model_sex = glue::glue("{model} ({str_to_title(sex)})"),
) %>%
arrange(model_sex) %>%
mutate(
model_sex = factor(model_sex,levels=order.model),
model_sex = fct_rev(model_sex),
)
}
magora_corrplot <- function(data,ran)
{
ggplot2::ggplot() +
ggplot2::geom_tile(data = data, ggplot2::aes(x = .data$module, y = .data$model_sex), colour = "black", fill = "white") +
ggplot2::geom_point(data = dplyr::filter(data), ggplot2::aes(x = .data$module, y = .data$model_sex, colour = .data$correlation, size = abs(.data$correlation))) +
ggplot2::geom_point(data = dplyr::filter(data, .data$significant), ggplot2::aes(x = .data$module, y = .data$model_sex, colour = .data$correlation),color="black",shape=0,size=9) +
ggplot2::scale_x_discrete(position = "top") +
ggplot2::scale_size(guide = "none", limits = c(0, ran)) +
ggplot2::scale_color_gradient2(limits = c(-ran, ran), breaks = c(-ran, 0, ran), low = "#85070C", high = "#164B6E", name = "Correlation", guide = ggplot2::guide_colorbar(ticks = FALSE)) +
ggplot2::labs(x = NULL, y = NULL) +
#ggplot2::ggtitle("Model(Sex)| Age/Control") +
ggplot2::facet_grid( rows = dplyr::vars(.data$age),cols = dplyr::vars(.data$cluster_label), scales = "free", space = "free",switch = 'y') +
ggplot2::theme(
strip.text.x = ggplot2::element_text(size = 10,colour = c("black")),
strip.text.y.left = ggplot2::element_text(angle = 0,size = 12),
axis.ticks = ggplot2::element_blank(),
axis.text.x = ggplot2::element_text(angle = 90, hjust = 0, size = 12),
axis.text.y = ggplot2::element_text(angle = 0, size = 12),
panel.background = ggplot2::element_blank(),
plot.title = ggplot2::element_text(angle = 0, vjust = -54, hjust = 0.03,size=12,face="bold"),
plot.title.position = "plot",
panel.grid = ggplot2::element_blank(),
legend.position = "right"
)
}
We can use these functions directly onto our other dataset from LOAD1 cohort.
Correlation between LOAD1 models and human AD modules
Reading Gene Expression Count matrix of LOAD1 cohort from Previous Lesson
load("../results/DEAnalysis_LOAD1.Rdata")
First, check the data:
head(DE_LOAD1.df)
symbol EntrezGene baseMean log2FoldChange lfcSE
ENSMUSG00000000001 Gnai3 14679 2587.33246 -0.027251874 0.05341582
ENSMUSG00000000028 Cdc45 12544 96.38913 -0.086316094 0.19815501
ENSMUSG00000000031 H19 14955 23.15992 -0.402670365 0.47978365
ENSMUSG00000000037 Scml2 107815 94.24871 -0.093235144 0.23666227
ENSMUSG00000000049 Apoh 11818 11.94380 -0.220259366 0.59500274
ENSMUSG00000000056 Narf 67608 3464.49361 0.009537733 0.05167193
stat pvalue padj model sex age
ENSMUSG00000000001 -0.5101836 0.6099228 0.9968456 APOE4 male 4
ENSMUSG00000000028 -0.4355989 0.6631278 0.9968456 APOE4 male 4
ENSMUSG00000000031 -0.8392749 0.4013151 0.9932419 APOE4 male 4
ENSMUSG00000000037 -0.3939586 0.6936116 0.9968456 APOE4 male 4
ENSMUSG00000000049 -0.3701821 0.7112468 0.9968456 APOE4 male 4
ENSMUSG00000000056 0.1845825 0.8535565 0.9968456 APOE4 male 4
unique(DE_LOAD1.df$model)
[1] "APOE4" "Trem2" "APOE4Trem2"
unique(DE_LOAD1.df$sex)
[1] "male" "female"
unique(DE_LOAD1.df$age)
[1] 4 8 12
Perform the correlation analysis
We directly input logFC table DE_LOAD1.df to corr_function:
order.model <- c("APOE4 (Male)", "APOE4 (Female)","Trem2 (Male)", "Trem2 (Female)","APOE4Trem2 (Male)", "APOE4Trem2 (Female)")
correlation_for_plot <- corr_function(DE_LOAD1.df)
head(correlation_for_plot)
# A tibble: 6 × 10
cluster cluster_label module model sex age correlation p_value significant
<chr> <fct> <fct> <chr> <chr> <int> <dbl> <dbl> <lgl>
1 Consen… "Consensus C… TCXbl… APOE4 fema… 4 -0.0251 3.37e-1 FALSE
2 Consen… "Consensus C… PHGye… APOE4 fema… 4 -0.148 2.34e-5 TRUE
3 Consen… "Consensus C… IFGye… APOE4 fema… 4 0.0911 1.86e-2 TRUE
4 Consen… "Consensus C… TCXbl… APOE4 fema… 8 -0.0230 3.79e-1 FALSE
5 Consen… "Consensus C… PHGye… APOE4 fema… 8 -0.110 1.73e-3 TRUE
6 Consen… "Consensus C… IFGye… APOE4 fema… 8 -0.0566 1.45e-1 FALSE
# ℹ 1 more variable: model_sex <fct>
Visualize the correlation plot
#range of correlation
range(correlation_for_plot$correlation)
[1] -0.2256401 0.1073370
magora_corrplot(correlation_for_plot,0.25)
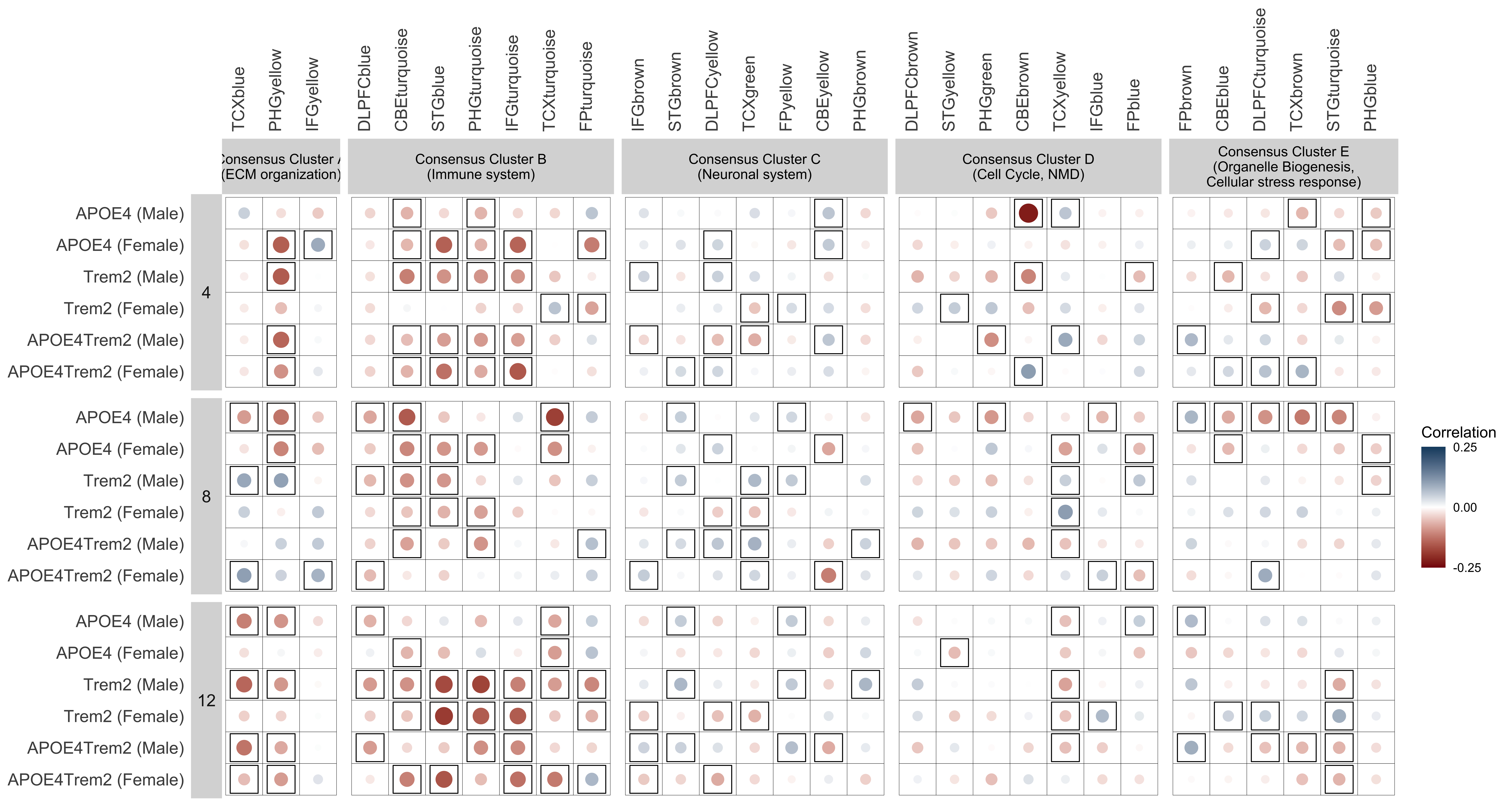
What do you conclude from this plot?
Key Points
AMP-AD gene modules represent major transcriptomic heterogeneity in AD.
Correlation of logFC is a practical approach for human-mouse alignment of AD-associated transcriptomic signatures.
Functional Alignment using AD Biological Domains
Overview
Teaching: 45 min
Exercises: 15 minQuestions
How to compare functional signatures from omics data between species?
Objectives
Understand how to detect changes in groups of related genes within a dataset.
Identify the common pitfalls of performing functional analyses.
Understand the source and utility of the biological domains of Alzheimer’s disease.
Use biological domain annotations to summarise and compare functional enrichments.
Author: Greg Cary, The Jackson Laboratory
Load libraries
suppressPackageStartupMessages(library("tidyverse"))
suppressPackageStartupMessages(library("org.Mm.eg.db"))
suppressPackageStartupMessages(library("org.Hs.eg.db"))
suppressPackageStartupMessages(library("clusterProfiler"))
suppressPackageStartupMessages(library("cowplot"))
suppressPackageStartupMessages(library("synapser"))
suppressPackageStartupMessages(library("ggridges"))
Detecting functional coherence of gene sets from omics data
Most omics analysis approaches produce data on many thousands of genomic features (i.e. transcripts, proteins, etc.) for each condition tested. Simply looking at these lists of genes and associated statistics can be daunting and uninformative. We need approaches to identify which biological functions are being impacted by a given experiment from these systems-level measurements.
Gene functional enrichment analysis describes a variety of statistical methods that identify groups of genes that share a particular biological function or process and show differential association with experimental conditions. Most approaches compare some statistically valid set of differentially expressed features to sets of functional annotations for those features. There are many different functional annotation sets available, some of the more commonly used include:
- gene function resources, such as the Gene Ontology (i.e. GO)
- pathway databases, such as Reactome or KEGG
- disease and phenotype ontologies, such as the Human Phenotype Ontology, the Mammalian Phenotype Ontology, and the Disease Ontology
These are the resources that are the foundation for many genomics knowledge bases, such as MGI, monarch initiative, and the Alliance of Genome Resources. The the precise nature of each of these resources varies, but the core information contained within each is the relationship of sets of genes to biologically meaningful annotations. These annotations are typically expertly curated from the published literature.
There are a variety of statistical approaches that can be employed to test for functional enrichment among genes identified from an omics dataset. Two of the most common broad classes of tests are over-representation analysis (ORA) and gene set enrichment analysis (GSEA). For example, consider the figure below from Zhao & Rhee, Trends in Genetics (2023). Let’s consider each in a bit more detail.
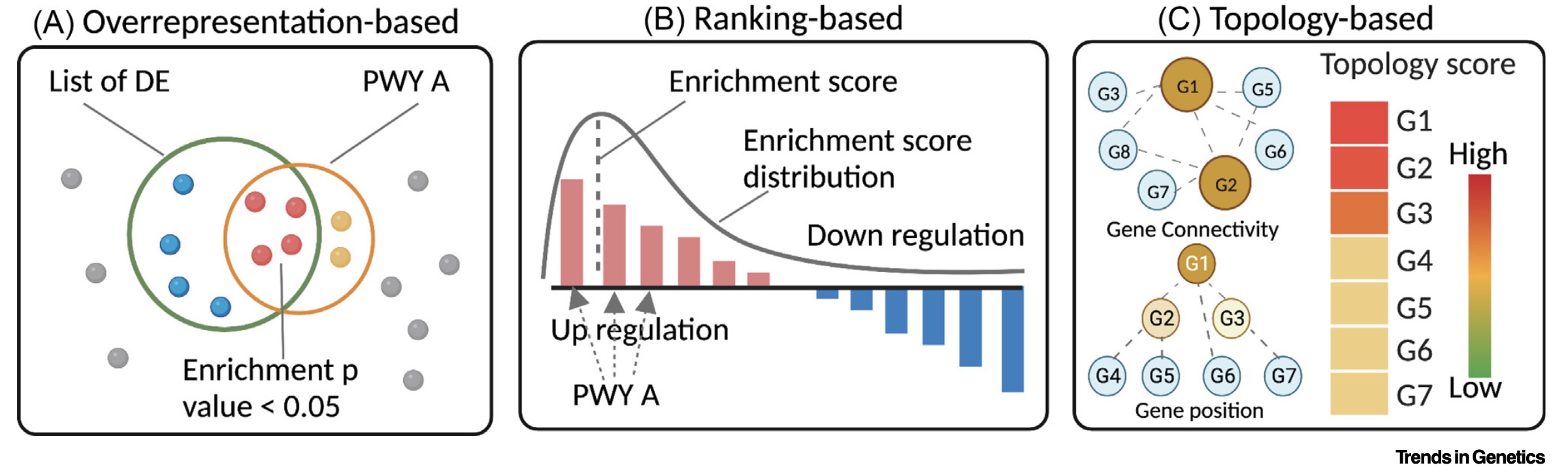
Over-representation analysis
ORA involves statistical tests of overlap between two lists of genes: one derived from the experiment and one from the functional annotations. For example, one might ask what is the overlap between the genes in an annotation class, such as “Lysosome”, and the genes that are significantly up-regulated in a given experimental condition. These tests usually rely on some form of Fisher’s exact test (e.g. fisher.test()) or hypergeometric test (e.g. phyper()). If the gene lists consist of a larger number of overlapping genes than would be expected at random - given the sample sizes involved - then there is said to be a statistical over-representation of the annotation class within the experimental condition.
Of course, these overlap tests are generally considered for all annotation classes, which can number in the hundreds to thousands. Performing this many statistical tests ensures that many will be identified as significant by chance. Therefore, there is typically a requirement to correct for multiple testing errors (e.g. p.adjust()).
There are many R packages available to handle the statistical tests and corrections involved in ORA. Today we’ll use clusterProfiler::enrichGO(), which wraps statistical testing for overlap with GO terms and multiple test correction in one function.
Let’s start by considering the enrichments from the mouse data analyzed previously.
theme_set(theme_bw())
load('../results/DEAnalysis_5XFAD.RData')
#load('../data/DEAnalysis_5XFAD.RData')
We’ll start by considering the genes that are significantly DE in 12 month old male animals
gene.list.up <- DE_5xFAD.df %>%
filter(sex == 'male',
age == '12 mo',
padj <= 0.05,
log2FoldChange > 0) %>%
arrange(desc(log2FoldChange)) %>%
filter(!duplicated(symbol), !is.na(symbol)) %>%
pull(symbol) %>%
unique()
gene.list.dn <- DE_5xFAD.df %>%
filter(sex == 'male',
age == '12 mo',
padj <= 0.05,
log2FoldChange < 0) %>%
arrange(desc(log2FoldChange)) %>%
filter(!duplicated(symbol), !is.na(symbol)) %>%
pull(symbol) %>%
unique()
length(gene.list.up)
[1] 1054
length(gene.list.dn)
[1] 491
There are a total of 1,055 significantly up-regulated genes and 491 significantly down-regulated genes in this cohort. Now test for over representation of GO terms among the DEGs. First, we need to identify the universe of all possible genes which includes the genes that were both measured by the experiment and contained within the annotation set.
univ <- as.data.frame(org.Mm.egGO) %>%
pull(gene_id) %>%
unique() %>%
bitr(., fromType = "ENTREZID", toType = 'SYMBOL', OrgDb = org.Mm.eg.db, drop = T) %>%
pull('SYMBOL') %>%
intersect(., DE_5xFAD.df$symbol)
'select()' returned 1:1 mapping between keys and columns
Now let’s test for enriched GO terms (this can take 3-4 minutes)
enr.up <- enrichGO(gene.list.up,
ont = 'all',
OrgDb = org.Mm.eg.db,
keyType = 'SYMBOL',
universe = univ
)
enr.dn <- enrichGO(gene.list.dn,
ont = 'all',
OrgDb = org.Mm.eg.db,
keyType = 'SYMBOL',
universe = univ
)
How many significant terms are identified:
enr.up@result %>% filter(p.adjust <= 0.05) %>% pull(ID) %>% unique() %>% length()
[1] 1597
enr.dn@result %>% filter(p.adjust <= 0.05) %>% pull(ID) %>% unique() %>% length()
[1] 531
Challenge 1
1) How many significant terms are identified from the up-regulated gene list if you do not specify the “universe”?
Solution to Challenge 1
enrichGO(gene.list.up, ont = 'all', OrgDb = org.Mm.eg.db, keyType = 'SYMBOL') %>% .@result %>% filter(p.adjust <= 0.05) %>% pull(ID) %>% unique() %>% length()
Plot the top 10 significant terms:
cowplot::plot_grid(
dotplot(enr.dn, showCategory = 10) + ggtitle('down'),
dotplot(enr.up, showCategory = 10) + ggtitle('up')
)
From this we can see that nervous system related terms (e.g. “learning or memory”, “neurotransmitter transport”) are down in 5xFAD males at 12 months, while immune functions (e.g. “cytokine-mediated signaling pathway” and “leukocyte mediated immunity”) are up in 5xFAD males at 12 months.
Gene set enrichment analysis
GSEA is an alternative approach that uses a statistical measure from the omics data (e.g. the log fold change or significance) to rank the genes. An “enrichment score” is calculated for each annotation set based on where the genes annotated to the term sit in the overall distribution.
Let’s analyze those same data with the clusterProfiler::gseGO() function. First we’ll specify the gene list and use the log2FoldChange value to rank the genes in the list.
gene.list <- DE_5xFAD.df %>%
filter(sex == 'male',
age == '12 mo',
padj <= 0.05
) %>%
arrange(desc(log2FoldChange)) %>%
filter(!duplicated(symbol), !is.na(symbol)) %>%
pull(log2FoldChange, name = symbol)
Now we’ll test for enrichment:
enr <- gseGO(gene.list, ont = 'all', OrgDb = org.Mm.eg.db, keyType = 'SYMBOL')
How many significant terms are identified:
enr@result %>% filter(p.adjust <= 0.05) %>% pull(ID) %>% unique() %>% length()
[1] 295
Challenge 2
1) The tally above represents all genes, both up- and down-regulatd. To compare between GSEA and ORA, can you identify how many GSEA enriched terms are associated with up-regulated genes and how many are associated with down-regulated genes? (Hint: the
NESvalue within the results relates to the directionality of enrichment).Solution to Challenge 2
enr@result %>% filter(p.adjust <= 0.05, NES < 0) %>% pull(ID) %>% unique() %>% length() enr@result %>% filter(p.adjust <= 0.05, NES > 0) %>% pull(ID) %>% unique() %>% length()
Plot the term enrichments
ridgeplot(enr, core_enrichment = T, showCategory = 20)
Picking joint bandwidth of 0.224
This plot shows the top 20 GSEA enriched terms. The displayed terms are all from the up-regulated class (i.e. NES > 0), and consist of many immune-relevant terms (e.g. “immune response” and “cytokine production”).
Common pitfalls & best practices
These kinds of functional enrichment analyses are very common, but not all results reported are equal! A recent paper describes an “Urgent need for consistent standards in functional enrichment analysis”{target=’_blank’}. They examine how the results of functional enrichment analyses are reported in the literature, and identify several common shortcomings. Watch out for these common mistakes when performing and reporting your own analyses!
Alzheimers’s Disease Biological Domains
While these results are informative and help to provide essential biological context to the results of the omics experiment, it is difficult to understand all of the enriched terms and what that means for the biology of disease. It would be useful to group each of the enriched terms within broader areas of disease biology. There are several common molecular endophenotypes that are detected within human Alzheimer’s cohorts across omics data types (transcriptomics, proteomics, and genetics). Several of these endophenotypic areas are shown in the figure below (source: Jesse Wiley).
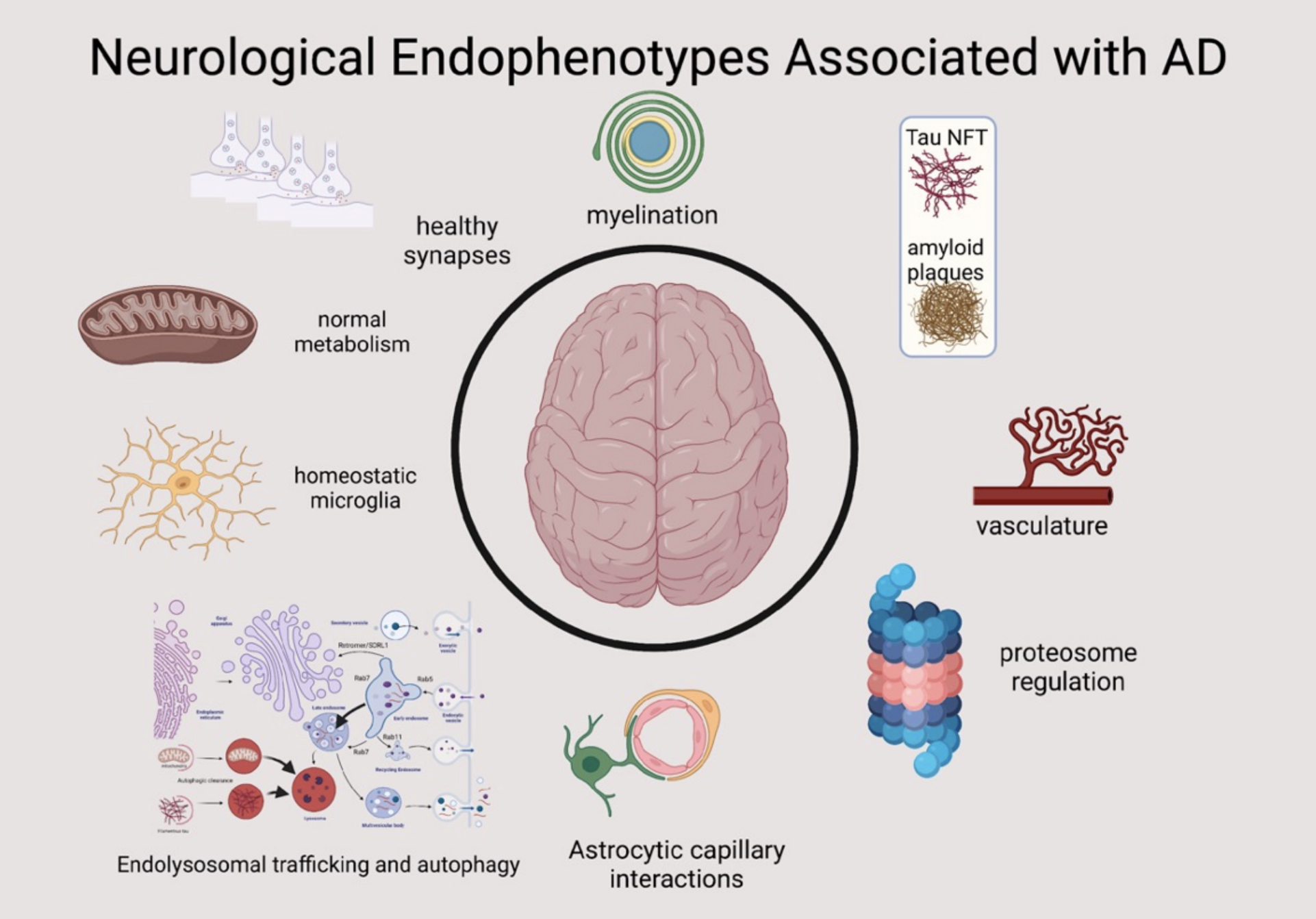
These endophenotypes describe molecular processes that are dysregulated by or during the disease process. Very similar sets of endophenotypes have been cataloged among the targets of therapeutics in clinical trials for AD (see below, Cummings et al, Alzheimer’s disease drug development pipeline: 2023, Alz Dem TRCI{target=’_blank’}).
In order to formalize and operationalize these endophenotypic areas, the Emory-Sage-SGC center of the TREAT-AD consortium has developed the biological domains of AD. In the enumeration of these domains, several criteria were established; the biological domains defined should be:
- objective: leverage existing well-annotated resources
- automatable: in CADRO, therapeutics and targets are manually assigned
- intelligible: groupings should be easy to understand
- modifiable: definitions should be (and are!) continuously be updated.
In all 19 distinct biological domains (aka biodoms) have been identified. These biological domains are defined using sets of GO terms that align with the endophenotype. For example, terms like “cytokine receptor binding” and “leukocyte migration” are annotated to the Immune Response biodomain, while terms like “postsynaptic density” and “synaptic vesicle cycle” are annotated to the Synapse biodomain. Of all terms in the GO, 7,127 terms (16.4%) are annotated to one of the biological domains. Because the GO terms have genes annotated, genes can be associate with specific endophenotypes via the biological domain groupings of terms. In all, 18,289 genes annotated to at least 1 biodom term. While the biodomains exhibit very few overlapping GO terms (panel A), due to gene pleiotropy (etc) the number of overlapping genes between biological domains is quite a bit higher (panel B). The development of the biological domains is described in more detail in the medRxiv preprint Genetic and Multi-omic Risk Assessment of Alzheimer’s Disease Implicates Core Associated Biological Domains.
Download and explore the biological domain annotations
First let’s download the biodomain definition file from synapse.
synLogin()
NULL
# biodomain definitions
biodom <- readRDS(synGet('syn25428992')$path)
dom.lab <- read_csv(synGet('syn26856828')$path)
Rows: 20 Columns: 4
── Column specification ───────────────────────────────────────────────────────────────────────
Delimiter: ","
chr (4): domain, abbr, label, color
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
What is in the file?
head(biodom)
# A tibble: 6 × 12
# Rowwise:
GO_ID GOterm_Name Biodomain n_symbol symbol uniprot n_ensGene ensembl_id
<chr> <chr> <chr> <int> <list> <list> <int> <list>
1 GO:0006955 immune resp… Immune R… 1544 <chr> <chr> 785 <chr>
2 GO:0019882 antigen pro… Immune R… 101 <chr> <chr> 151 <chr>
3 GO:0002376 immune syst… Immune R… 2165 <chr> <chr> 1215 <chr>
4 GO:0045087 innate immu… Immune R… 733 <chr> <chr> 799 <chr>
5 GO:0006954 inflammator… Immune R… 537 <chr> <chr> 490 <chr>
6 GO:0002250 adaptive im… Immune R… 585 <chr> <chr> 733 <chr>
# ℹ 4 more variables: n_entrezGene <int>, entrez_id <list>, n_hgncSymbol <int>,
# hgnc_symbol <list>
You can see the file is a list of GO term accessions (GO_ID) and names (GOterm_Name) as well as the corresponding endophenotype (Biodomain) to which the term is annotated. There are also gene annotations for each term. These annotation sets come from two sources: (1) the symbol and uniprot annotations are drawn directly from the Gene Ontology via the provided API, (2) ensembl_id, entrez_id, and hgnc_symbol are from BioMart annotations (e.g. biomaRt::getLDS()).
We can see how many GO terms are annotated to each biodomain:
biodom %>% group_by(Biodomain) %>% summarise(n_term = length(unique(GO_ID))) %>% arrange(n_term)
# A tibble: 19 × 2
Biodomain n_term
<chr> <int>
1 Tau Homeostasis 10
2 APP Metabolism 37
3 RNA Spliceosome 51
4 Myelination 66
5 Oxidative Stress 98
6 Autophagy 112
7 DNA Repair 122
8 Apoptosis 218
9 Endolysosome 236
10 Metal Binding and Homeostasis 301
11 Cell Cycle 320
12 Vasculature 374
13 Epigenetic 432
14 Structural Stabilization 498
15 Mitochondrial Metabolism 532
16 Proteostasis 758
17 Lipid Metabolism 875
18 Immune Response 979
19 Synapse 1379
The biodomains range in size from Tau Homeostasis (10 terms) up to Synapse (1,379 terms).
We can also investigate the individual genes annotated to each biodomain term.
biodom %>% filter(GOterm_Name == 'amyloid-beta formation') %>% pull(symbol) %>% unlist()
[1] "PSEN1" "APH1B" "ABCA7" "BACE1" "DYRK1A" "NCSTN" "PSEN2" "PSENEN"
[9] "APH1A"
So the genes associated with amyloid-beta formation within the APP Metabolism biodomain include PSEN1, PSEN2, BACE1, ABCA7, NCSTN, and others.
Annotate enriched terms with biological domain
Let’s re-characterize the 5xFAD functional enrichments we computed previously with the biological domain annotations and see if we can get more context about what is changing in that model. We’ll focus first on the GSEA results and start by annotating the results with biodomain groupings.
enr.bd = enr@result %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain)
head(enr.bd[,1:4])
Biodomain ONTOLOGY ID Description
1 Immune Response BP GO:0002376 immune system process
2 Immune Response BP GO:0006955 immune response
3 <NA> BP GO:0006952 defense response
4 <NA> BP GO:0002682 regulation of immune system process
5 <NA> BP GO:0009607 response to biotic stimulus
6 <NA> BP GO:0043207 response to external biotic stimulus
Not all of the enriched terms are annotated to a biological domain. Some terms are too broad and not specific (e.g. ‘defense response’), while others may not have been captured by a biological domain annotation yet (e.g. ‘regulation of immune system process’). Remember that the conception of the biodomains involved a requirement that they be modifiable, and these terms may be added to the biodomain in the future. Let’s modify the Biodomain value for terms that aren’t annotated to a domain to ‘none’.
enr.bd$Biodomain[is.na(enr.bd$Biodomain)] <- 'none'
head(enr.bd[,1:4])
Biodomain ONTOLOGY ID Description
1 Immune Response BP GO:0002376 immune system process
2 Immune Response BP GO:0006955 immune response
3 none BP GO:0006952 defense response
4 none BP GO:0002682 regulation of immune system process
5 none BP GO:0009607 response to biotic stimulus
6 none BP GO:0043207 response to external biotic stimulus
How many terms are enriched from each domain?
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
arrange(bd.tally, desc(n_sig_term))
# A tibble: 20 × 3
# Rowwise:
domain n_term n_sig_term
<chr> <int> <int>
1 none 0 141
2 Immune Response 979 122
3 Structural Stabilization 498 8
4 Apoptosis 218 5
5 Synapse 1379 5
6 Autophagy 112 4
7 Proteostasis 758 4
8 Lipid Metabolism 875 3
9 Vasculature 374 1
10 Myelination 66 1
11 Mitochondrial Metabolism 532 1
12 Endolysosome 236 0
13 Epigenetic 432 0
14 Oxidative Stress 98 0
15 APP Metabolism 37 0
16 Tau Homeostasis 10 0
17 RNA Spliceosome 51 0
18 Cell Cycle 320 0
19 Metal Binding and Homeostasis 301 0
20 DNA Repair 122 0
Many enriched terms don’t map to a domain (134), but most do (154). Of those that do, the vast majority map into the Immune Response biodomain.
We can plot the enrichment results, stratified by biodomain:
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
mutate(Biodomain = factor(Biodomain, levels = arrange(bd.tally, n_sig_term) %>% pull(domain))) %>%
arrange(Biodomain, p.adjust)
ggplot(enr.bd, aes(NES, Biodomain)) +
geom_violin(data = subset(enr.bd, NES > 0),
aes(color = color), scale = 'width')+
geom_violin(data = subset(enr.bd, NES < 0),
aes(color = color), scale = 'width')+
geom_jitter(aes(size = -log10(p.adjust), fill = color),
color = 'grey20', shape = 21, alpha = .3)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
scale_y_discrete(drop = F)+
scale_fill_identity()+scale_color_identity()
Warning: Groups with fewer than two data points have been dropped.
Groups with fewer than two data points have been dropped.
Groups with fewer than two data points have been dropped.
Groups with fewer than two data points have been dropped.
Groups with fewer than two data points have been dropped.
Warning: Removed 9 rows containing missing values (`geom_point()`).
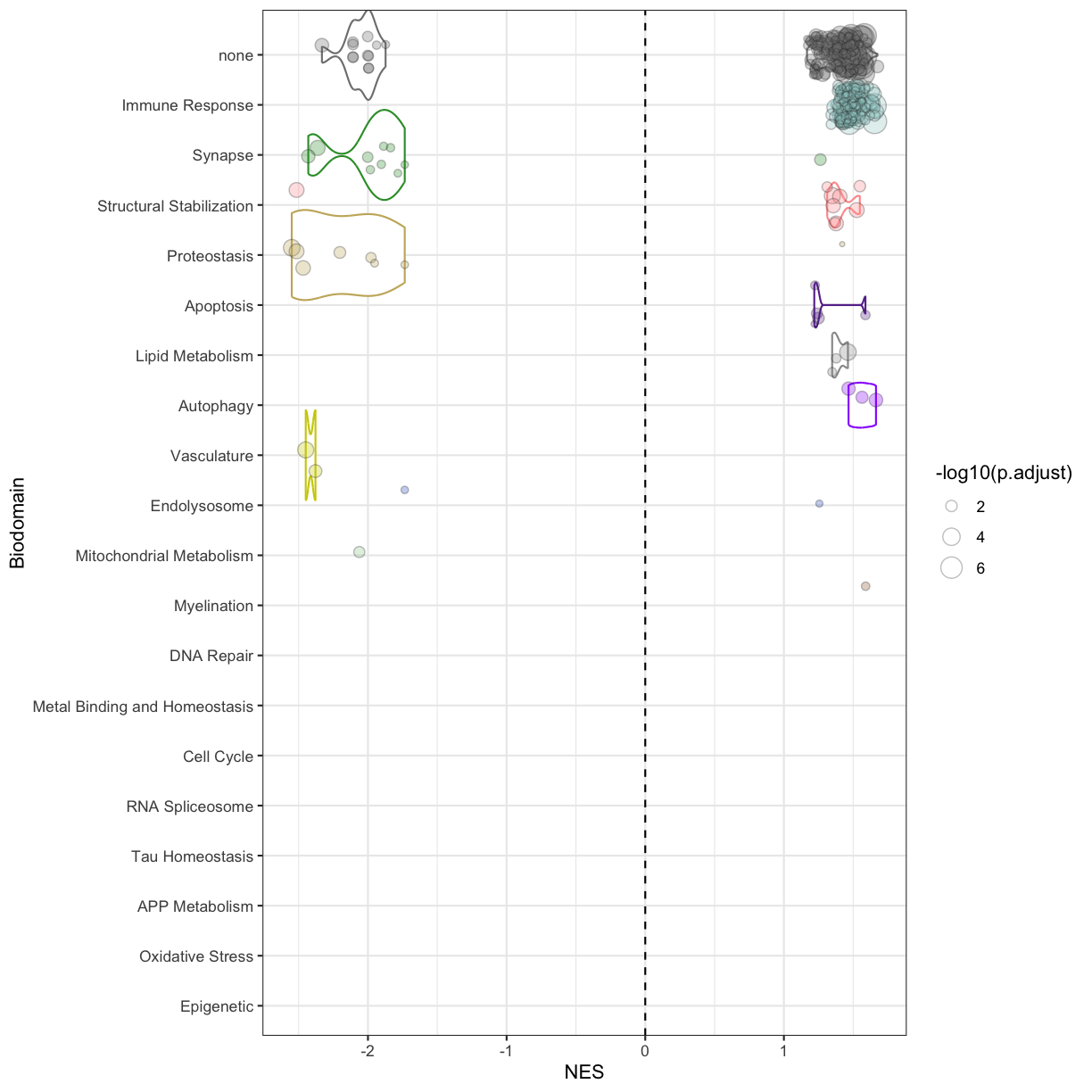
plot of chunk enrichment_results
We can produce a similar plot for the ORA results. Let’s combine the up and down analyses into one, and make the adjusted p-value signed so that we can look at terms that are up and down separately:
enr.ora = bind_rows(
enr.up@result %>% mutate(dir = 'up'),
enr.dn@result %>% mutate(dir = 'dn')
) %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain)
enr.ora$Biodomain[is.na(enr.ora$Biodomain)] <- 'none'
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.ora$ID[ enr.ora$Biodomain == domain ] %>% unique() %>% length()
)
enr.ora <- full_join(enr.ora, dom.lab, by = c('Biodomain' = 'domain')) %>%
mutate(Biodomain = factor(Biodomain, levels = arrange(bd.tally, n_sig_term) %>% pull(domain))) %>%
arrange(Biodomain, p.adjust) %>%
mutate(signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'dn', -1*signed_logP, signed_logP))
ggplot(enr.ora, aes(signed_logP, Biodomain)) +
geom_violin(data = subset(enr.ora, dir == 'up'), aes(color = color), scale = 'width')+
geom_violin(data = subset(enr.ora, dir == 'dn'), aes(color = color), scale = 'width')+
geom_jitter(aes(size = Count, fill = color), color = 'grey20', shape = 21, alpha = .3)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
scale_y_discrete(drop = F)+
scale_fill_identity()+scale_color_identity()
plot of chunk ORA_results
Because there are many more significantly enriched terms from the ORA, there is more detail to consider. The dominant signal is stil the up-regulation of terms from the Immune Response biodomain. There is also nearly exclusive up-regulation of terms from the Autophagy, Metal Binding and Homeostasis, Oxidative Stress, and APP Metabolism domains. The most down-regulated terms are from the Synapse biodomain.
Challenge 3
1) While there are many more terms identified using ORA compared with GSEA, how many Biodomain terms are identified in both analyses vs unique to each? 2) Which biodomains and terms are uniquely identified in the GSEA analysis?
Solution to Challenge 3
1).
intersect(enr.bd$Description[enr.bd$Biodomain!='none'], enr.ora$Description[enr.ora$Biodomain!='none']) %>% length()133 in common
setdiff(enr.bd$Description[enr.bd$Biodomain!='none'], enr.ora$Description[enr.ora$Biodomain!='none']) %>% length()20 unique to GSEA
setdiff(enr.ora$Description[enr.ora$Biodomain!='none'], enr.bd$Description[enr.bd$Biodomain!='none']) %>% length()908 unique to ORA
2).
enr.bd %>% filter( Description %in% setdiff(enr.bd$Description[enr.bd$Biodomain!='none'], enr.ora$Description[enr.ora$Biodomain!='none']) ) %>% group_by(Biodomain) %>% summarise(terms = paste0(Description, collapse = ', '))Most terms unique to GSEA are from the ‘Immune Response’ domain (13), but there are also terms from ‘Structural Stabilization’ (4), ‘Apoptosis’ (2), and ‘Synapse’ (1) that are unique to the GSEA results.
Biological domains over model age
Let’s take a look at how biodomain enrichments from the MODEL-AD strains change with respect to the age of the model. To save some time, let’s load some pre-computed enrichment results for mouse & human data.
mm.ora <- bind_rows(
read_tsv('../data/5xFAD_ora_results.tsv', col_types = cols()) %>%
mutate(age = str_remove_all(age, ' mo') %>% as.double()),
read_tsv('../data/LOAD1_ora_results.tsv', col_types = cols())
)
mm.gsea <- bind_rows(
read_tsv('../data/5xFAD_gsea_results.tsv', col_types = cols()) %>%
mutate(age = str_remove_all(age, ' mo') %>% as.double()),
read_tsv('../data/LOAD1_gsea_results.tsv', col_types = cols())
)
hs.ora <- read_tsv('../data/Hsap_ora_results.tsv', col_types = cols())
hs.gsea <- read_tsv('../data/Hsap_gsea_results.tsv', col_types = cols())
Annotate and plot the 5xFAD Male results, this time faceting by biodomain to compare across the age groups:
enr.bd = mm.ora %>%
filter(model == '5xFAD', sex == 'male') %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain) %>%
mutate(Biodomain = if_else( is.na(Biodomain), 'none', Biodomain),
signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'ora_dn', -1*signed_logP, signed_logP))
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
mutate(age = factor(age, enr.bd$age %>% unique() %>% sort(decreasing = T) ),
Biodomain = factor(Biodomain, levels = arrange(bd.tally, desc(n_sig_term)) %>% pull(domain))) %>%
arrange(Biodomain, p.adjust)
ggplot(enr.bd, aes(signed_logP, age)) +
geom_violin(data = subset(enr.bd, signed_logP > 0), aes(color = color), scale = 'width')+
geom_violin(data = subset(enr.bd, signed_logP < 0), aes(color = color), scale = 'width')+
geom_point(aes(size = Count, fill = color, group = ID),
color = 'grey20', shape = 21, alpha = .5, position = position_jitter(width = .25, seed = 2) )+
facet_wrap(~Biodomain)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
scale_fill_identity()+scale_color_identity()
plot of chunk Male_5xFAD_biodomain
The Immune Response terms are over-represented among the up-regulated genes at all ages, but the overall significance of the associations increase with age. Similar age-dependent trends are apparent in other domains as well. Of note is the increasing number of terms from the Synapse domain over-represented among the down-regulated genes as the animals age.
Let’s consider the APOE4Trem2 results. Annotate the biodomains and plot the results of ORA from the DE genes in APOE4Trem2 Males:
enr.bd = mm.ora %>%
filter(model == 'APOE4Trem2', sex == 'male') %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain) %>%
mutate(Biodomain = if_else( is.na(Biodomain), 'none', Biodomain),
signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'ora_dn', -1*signed_logP, signed_logP))
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
filter(!is.na(age)) %>%
mutate(age = factor(age, levels = enr.bd$age %>% unique() %>% sort(decreasing = T)),
Biodomain = factor(Biodomain,
levels = arrange(bd.tally, desc(n_sig_term)) %>% pull(domain))) %>%
arrange(age, desc(Biodomain), p.adjust)
ggplot(enr.bd, aes(signed_logP, age)) +
geom_violin(data = subset(enr.bd, signed_logP > 0), aes(color = color), scale = 'width')+
geom_violin(data = subset(enr.bd, signed_logP < 0), aes(color = color), scale = 'width')+
geom_jitter(aes(size = Count, fill = color), color = 'grey20', shape = 21, alpha = .5)+
facet_wrap(~Biodomain)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
scale_fill_identity()+scale_color_identity()
plot of chunk APOE4Trem2_biodomain
There aren’t any terms enriched at the 4 month time point. Unlike the 5xFAD mice, APOE4Trem2 mice show a significant down-regulation of Immune Response terms, particularly at 8 months, as well as down-regulation in terms from APP Metabolism domain. There is an enrichment in terms from the Synapse domain for down-regulated genes, despite enrichments among down-regulated genes in cell death related domains Autophagy and Apoptosis.
Challenge 4
Terms from
Immune Responseare enriched among the genes up in 5xFAD, but down in APOE4Trem2. Are these similar or different terms in each set? Focus on the 12 month old animals from each model.Solution to Challenge 4
First, get the list of terms associated with up- and down-regulated genes in each model:
xf = mm.ora %>% filter(model == '5xFAD', sex == 'male', age == 12, dir == 'ora_up') %>% left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>% filter(Biodomain == 'Immune Response') %>% pull(Description) at = mm.ora %>% filter(model == 'APOE4Trem2', sex == 'male', age == 12, dir == 'ora_dn') %>% left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>% filter(Biodomain == 'Immune Response') %>% pull(Description)Next how many terms are in each list and how many terms overlap?
length(xf) length(at) length(intersect(at,xf))Almost all terms (95.5%) enriched in the down-regulated analysis from APOE4Trem2 are also found in the enrichments from the up-regulated genes in 5xFAD.
Comparing Human and Mouse functional enrichments
Finally let’s consider how we can compare the functional enrichments in the mouse models with functional enrichments from the AMP-AD cohorts. We can first take a look at the functional enrichments from the different human cohorts.
The cohorts ans tissues available are:
hs.ora %>% select(Study, Tissue) %>% distinct()
# A tibble: 6 × 2
Study Tissue
<chr> <chr>
1 MAYO CBE
2 MAYO TCX
3 MSSM STG
4 MSSM PHG
5 MSSM IFG
6 ROSMAP DLPFC
We can focus on one tissue per cohort, let’s look at TCX for Mayo, PHG for Mt Sinai, and DLPFC for ROSMAP.
enr.bd = hs.ora %>%
filter(Tissue %in% c('TCX','PHG','DLPFC')) %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain) %>%
mutate(Biodomain = if_else( is.na(Biodomain), 'none', Biodomain),
signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'ora_dn', -1*signed_logP, signed_logP))
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
filter(!is.na(Study)) %>%
mutate(Biodomain = factor(Biodomain, levels = arrange(bd.tally, desc(n_sig_term)) %>% pull(domain))) %>%
arrange(Biodomain, p.adjust)
ggplot(enr.bd, aes(signed_logP, Biodomain)) +
geom_violin(data = subset(enr.bd, signed_logP > 0), aes(color = color), scale = 'width')+
geom_violin(data = subset(enr.bd, signed_logP < 0), aes(color = color), scale = 'width')+
geom_point(aes(size = Count, fill = color, group = ID),
color = 'grey20', shape = 21, alpha = .5, position = position_jitter(width = .25, seed = 2) )+
facet_wrap(~paste0(Study, ': ', Tissue))+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
scale_fill_identity()+scale_color_identity()
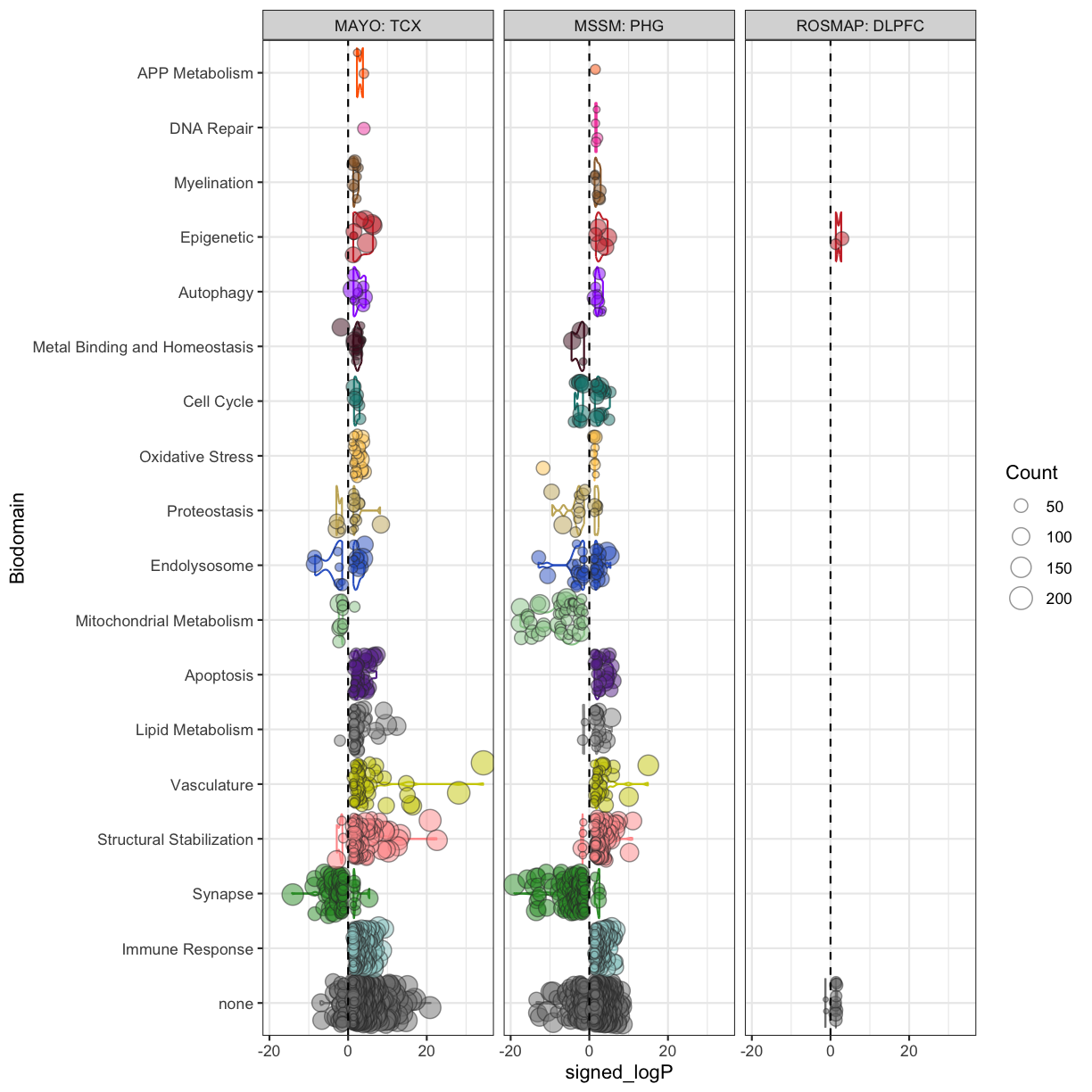
plot of chunk biodomain_one_tissue_per_cohort
Huh, the Mayo and MSSM look largely similar, but the ROSMAP DLPFC doesn’t have very many enriched terms. Let’s ignore the ROSMAP result for now and compare the terms enriched between Mayo TCX and the 5xFAD males. First we can join up the enriched terms between the sets into one data frame.
comb_5x = bind_rows(
hs.ora %>%
filter(Study == 'MAYO', Tissue == 'TCX') %>%
mutate(species = 'hs') %>%
select(ID, Description, species, dir, p.adjust, Count),
mm.ora %>%
filter(model == '5xFAD', sex == 'male', age == 12) %>%
mutate(species = 'mm') %>%
select(ID, Description, species, dir, p.adjust, Count)) %>%
mutate(signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'ora_dn', -1*signed_logP, signed_logP)) %>%
pivot_wider(id_cols = c(ID, Description),
names_from = species,
values_from = signed_logP
) %>%
unnest(cols = c(hs,mm)) %>%
mutate(across(c(hs,mm), ~ if_else(is.na(.x), 0, .x)))
Warning: Values from `signed_logP` are not uniquely identified; output will contain list-cols.
• Use `values_fn = list` to suppress this warning.
• Use `values_fn = {summary_fun}` to summarise duplicates.
• Use the following dplyr code to identify duplicates.
{data} %>%
dplyr::group_by(ID, Description, species) %>%
dplyr::summarise(n = dplyr::n(), .groups = "drop") %>%
dplyr::filter(n > 1L)
head(comb_5x)
# A tibble: 6 × 4
ID Description hs mm
<chr> <chr> <dbl> <dbl>
1 GO:0048514 blood vessel morphogenesis 34.3 0
2 GO:0001525 angiogenesis 28.0 0
3 GO:0009611 response to wounding 16.8 0
4 GO:0001503 ossification 16.2 2.00
5 GO:1901342 regulation of vasculature development 16.2 10.1
6 GO:0045765 regulation of angiogenesis 16.1 10.3
Great! Now let’s annotate the biodomains and plot it up:
enr.bd <- comb_5x %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain) %>%
mutate(Biodomain = if_else( is.na(Biodomain), 'none', Biodomain))
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
mutate(Biodomain = factor(Biodomain,
levels = arrange(bd.tally, desc(n_sig_term)) %>% pull(domain))) %>%
arrange(Biodomain)
ggplot(enr.bd, aes(hs, mm)) +
geom_point(aes(fill = color),color = 'grey20', shape = 21, alpha = .5)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
geom_hline(yintercept = 0, lwd = .5, lty = 2)+
facet_wrap(~Biodomain, scales = 'free')+
scale_fill_identity()
plot of chunk annotated_biodomains
There are several terms from multiple biodomains that are enriched in similar directions (i.e. from the up-regulated genes in both human AD and 5xFAD). There are fewer that are enriched in opposite directions (i.e. up in human AD and down in 5xFAD), but there are many terms that are unique to either the human or the mouse data set.
What if we compare the APOE4Trem2 males to Mayo TCX?
comb_at = bind_rows(
hs.ora %>%
filter(Study == 'MAYO', Tissue == 'TCX') %>%
mutate(species = 'hs') %>%
select(ID, Description, species, dir, p.adjust, Count),
mm.ora %>%
filter(model == 'APOE4Trem2', sex == 'male', age == 12) %>%
mutate(species = 'mm') %>%
select(ID, Description, species, dir, p.adjust, Count)) %>%
mutate(signed_logP = -log10(p.adjust),
signed_logP = if_else(dir == 'ora_dn', -1*signed_logP, signed_logP)) %>%
pivot_wider(id_cols = c(ID, Description),
names_from = species,
values_from = signed_logP
) %>%
unnest(cols = c(hs,mm)) %>%
mutate(across(c(hs,mm), ~ if_else(is.na(.x), 0, .x)))
enr.bd <- comb_at %>%
left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>%
relocate(Biodomain) %>%
mutate(Biodomain = if_else( is.na(Biodomain), 'none', Biodomain))
bd.tally = tibble(domain = c(unique(biodom$Biodomain), 'none')) %>%
rowwise() %>%
mutate(
n_term = biodom$GO_ID[ biodom$Biodomain == domain ] %>% unique() %>% length(),
n_sig_term = enr.bd$ID[ enr.bd$Biodomain == domain ] %>% unique() %>% length()
)
enr.bd <- full_join(enr.bd, dom.lab, by = c('Biodomain' = 'domain')) %>%
mutate(Biodomain = factor(Biodomain,
levels = arrange(bd.tally, desc(n_sig_term)) %>% pull(domain))) %>%
arrange(Biodomain)
ggplot(enr.bd, aes(hs, mm)) +
geom_point(aes(fill = color),color = 'grey20', shape = 21, alpha = .5)+
geom_vline(xintercept = 0, lwd = .5, lty = 2)+
geom_hline(yintercept = 0, lwd = .5, lty = 2)+
facet_wrap(~Biodomain, scales = 'free')+
scale_fill_identity()
Warning: Removed 1 rows containing missing values (`geom_point()`).
plot of chunk APOE4Trem2_MayoTCX
Many of the terms that are enriched among the down-regulated genes from APOE4Trem2 are enriched from genes up-regulated in AD, especially from Immune Response and Lipid Metabolism domains.
Overall, by aligning human and mouse omics signatures through the lens of domains affected in each context, we can get a better understanding of the relationships between the biological processes affected in each context.
Challenge 5
We haven’t focused on it much, but there are terms that aren’t in a biodomain and are enriched in opposite direction between the human and mouse model tests. Are there any terms that aren’t annotated to a biodomain, but are up in humand and down in both animal model tests?
Solution to Challenge 5
First, get the list of terms associated with up- and down-regulated genes in each model:
intersect( comb_5x %>% left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>% filter(is.na(Biodomain), hs > 0, mm < 0) %>% pull(Description), comb_at %>% left_join(., biodom %>% select(Biodomain, ID = GO_ID), by = 'ID') %>% filter(is.na(Biodomain), hs > 0, mm < 0) %>% pull(Description) )Intriguingly there are some apparently relevant terms, such as “negative regulation of gliogenesis”, that are up in human cohort and down in both mouse models. The biological domain annotations are a helpful tool, but certainly not the whole picture.
Session Info
sessionInfo()
R version 4.3.0 (2023-04-21)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Monterey 12.6.2
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.3-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.11.0
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: America/New_York
tzcode source: internal
attached base packages:
[1] stats4 stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] ggridges_0.5.4 synapser_1.0.59 cowplot_1.1.1
[4] clusterProfiler_4.8.1 org.Hs.eg.db_3.17.0 org.Mm.eg.db_3.17.0
[7] AnnotationDbi_1.62.1 IRanges_2.34.0 S4Vectors_0.38.1
[10] Biobase_2.60.0 BiocGenerics_0.46.0 lubridate_1.9.2
[13] forcats_1.0.0 stringr_1.5.0 dplyr_1.1.2
[16] purrr_1.0.1 readr_2.1.4 tidyr_1.3.0
[19] tibble_3.2.1 ggplot2_3.4.2 tidyverse_2.0.0
[22] knitr_1.43
loaded via a namespace (and not attached):
[1] DBI_1.1.3 bitops_1.0-7 gson_0.1.0
[4] shadowtext_0.1.2 gridExtra_2.3 rlang_1.1.1
[7] magrittr_2.0.3 DOSE_3.26.1 compiler_4.3.0
[10] RSQLite_2.3.1 png_0.1-8 vctrs_0.6.2
[13] reshape2_1.4.4 pkgconfig_2.0.3 crayon_1.5.2
[16] fastmap_1.1.1 XVector_0.40.0 labeling_0.4.2
[19] ggraph_2.1.0 utf8_1.2.3 HDO.db_0.99.1
[22] tzdb_0.4.0 enrichplot_1.20.0 bit_4.0.5
[25] xfun_0.39 zlibbioc_1.46.0 cachem_1.0.8
[28] aplot_0.1.10 jsonlite_1.8.5 GenomeInfoDb_1.36.0
[31] blob_1.2.4 highr_0.10 BiocParallel_1.34.2
[34] tweenr_2.0.2 parallel_4.3.0 R6_2.5.1
[37] stringi_1.7.12 RColorBrewer_1.1-3 reticulate_1.28
[40] GOSemSim_2.26.0 Rcpp_1.0.10 downloader_0.4
[43] Matrix_1.5-4 splines_4.3.0 igraph_1.4.3
[46] timechange_0.2.0 tidyselect_1.2.0 qvalue_2.32.0
[49] viridis_0.6.3 codetools_0.2-19 lattice_0.21-8
[52] plyr_1.8.8 treeio_1.24.1 withr_2.5.0
[55] KEGGREST_1.40.0 evaluate_0.21 gridGraphics_0.5-1
[58] scatterpie_0.2.1 polyclip_1.10-4 Biostrings_2.68.1
[61] ggtree_3.8.0 pillar_1.9.0 ggfun_0.0.9
[64] generics_0.1.3 vroom_1.6.3 rprojroot_2.0.3
[67] RCurl_1.98-1.12 hms_1.1.3 tidytree_0.4.2
[70] munsell_0.5.0 scales_1.2.1 glue_1.6.2
[73] lazyeval_0.2.2 tools_4.3.0 data.table_1.14.8
[76] fgsea_1.26.0 graphlayouts_1.0.0 fastmatch_1.1-3
[79] tidygraph_1.2.3 grid_4.3.0 ape_5.7-1
[82] colorspace_2.1-0 nlme_3.1-162 patchwork_1.1.2
[85] GenomeInfoDbData_1.2.10 ggforce_0.4.1 cli_3.6.1
[88] fansi_1.0.4 viridisLite_0.4.2 gtable_0.3.3
[91] yulab.utils_0.0.6 digest_0.6.31 ggrepel_0.9.3
[94] ggplotify_0.1.0 rjson_0.2.21 farver_2.1.1
[97] memoise_2.0.1 lifecycle_1.0.3 here_1.0.1
[100] httr_1.4.6 GO.db_3.17.0 bit64_4.0.5
[103] MASS_7.3-58.4
Key Points
Functional signatures are a useful point of comparison between species.
Subsetting functions into associated Biological Domains allows for more granular comparisons.